Human Papillomavirus (HPV) Vaccine Market Overview
HPV vaccine is a preventative measure against the Human Papillomavirus (HPV), a common sexually transmitted infection that can cause genital warts and various forms of cancer. HPV is spread through sexual intercourse and is classified into high-risk and low-risk strains. High-risk strains are linked to several types of cancer, such as cervical cancer. Most HPV infections show no symptoms and resolve without treatment, but long-lasting infections can eventually result in cancer. Routine screening and vaccination are crucial techniques for handling health risks associated with HPV, supported by major health organizations such as the WHO.
The growing use of vaccines to help lower the risk of cervical and other cancers caused by high-risk HPV strains is propelling the demand for the human papillomavirus (HPV) vaccine market. HPV vaccine assists the immune system in combatting the disease before it can be harmful, thereby reducing the chances of developing genital warts and cancers. The market is also witnessing increased vaccination efforts that target preteens and young adults to achieve the greatest impact in halting the spread of HPV and enhancing overall public health. Moreover, the rising advancements in vaccine formulations and the growing focus on preventive healthcare are likely to impact the market dynamics.
Human Papillomavirus (HPV) Vaccine Market Growth Drivers
Rising Incidence of HPV-Related Disease Spurs Market Growth
The growth in the HPV vaccine market is mainly being fueled by the rising cases of HPV-linked illnesses such as cervical, anal, and oropharyngeal cancers. In the United States, around 47,984 new cases of cancer related to human papillomavirus (HPV) are identified annually, with 26,280 cases in females and 21,704 in males. HPV causes about 37,800 of these cancers, with cervical cancer prevalent among women and oropharyngeal cancers in men. Further, as a common sexually transmitted infection (STI), HPV poses a significant risk of developing cancer. This has prompted global health organizations such as the WHO and CDC to advocate for widespread vaccination as a preventive measure, which is poised to boost market growth.Advancements in Vaccines Drive Increased Demand in the Human Papillomavirus (HPV) Vaccine Market
Advancements in HPV vaccine technology, including Gardasil 9 which guards against nine strains, have increased their ability to prevent different types of cancers. In September 2024, Merck announced positive top-line results from a Phase 3 trial evaluating the efficacy and safety of its 9-valent Human Papillomavirus (HPV) vaccine GARDASIL®9 in Japanese males aged 16 to 26. Continuing studies on single-shot vaccines and mixed vaccinations seek to streamline immunization procedures and improve adherence, particularly in areas with limited resources. Thus, the market is ready for further expansion as healthcare providers and governments implement these advanced vaccines to address the worldwide HPV disease burden.Human Papillomavirus (HPV) Vaccine Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:
Introduction of Multivalent Vaccines to Impact Market Growth
The introduction of multivalent HPV vaccines is a significant advancement in preventing HPV-related diseases. These vaccines offer protection against multiple strains, increasing effectiveness and simplifying immunization strategies. Widely adopting multivalent vaccines is expected to reduce HPV-related cancer rates and strengthen vaccination as a crucial preventive measure in global public health initiatives.Development of Combination Vaccines is Likely to Augment Human Papillomavirus (HPV) Vaccine Market Demand
The HPV vaccine market is seeing a rise in combination vaccines designed to protect against multiple infections, simplifying vaccination schedules and increasing patient compliance, especially in pediatric and adolescent populations. Ongoing research is exploring combinations of HPV immunization with protection against other STIs, such as Hepatitis B and HIV, offering broader protection with a single shot.Increasing HPV Vaccination in Low-Income Settings to Boost Human Papillomavirus (HPV) Vaccine Market Size
Efforts to increase HPV vaccination rates in low-income settings are expanding due to the high burden of HPV-related diseases, especially cervical cancer. Global organizations are working with governments to make HPV vaccines more accessible in low-resource areas. Strategies include subsidies, donations, tailored vaccination programs, school campaigns, and partnerships with health workers. These efforts aim to reduce disparities in cervical cancer deaths and promote global health goals for cancer prevention.Digital Health Tools for Vaccine Monitoring Set to Elevate Human Papillomavirus (HPV) Vaccine Market Value
Digital health tools are revolutionizing HPV vaccine monitoring and improving vaccination program effectiveness. Platforms track coverage, and appointments, and provide reminders to patients and providers in real time. These tools aid in data-driven decision-making for public health campaigns, ensuring timely vaccination doses. Digital technologies also improve provider-patient communication, increasing awareness and compliance for optimal vaccination strategies in the expanding HPV vaccine market.Human Papillomavirus (HPV) Vaccine Market Segmentation
The report titled “Human Papillomavirus (HPV) Vaccine Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Bivalent
- Polyvalent
Market Breakup by Indication
- Genital Warts
- Cervical Cancer
- Anal Cancer
- Penile Cancer
- Oropharyngeal Cancer
- Others
Market Breakup by Route of Administration
- Intravenous
- Intramuscular
- Others
Market Breakup by Distribution Channel
- Hospital and Retail Pharmacies
- Government Suppliers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Human Papillomavirus (HPV) Vaccine Market Share
Market Segmentation Based on the Type Holds Substantial Market Share
Based on type, the market is segmented into bivalent and polyvalent. Among these, the polyvalent segment is expected to dominate the market due to its broader protection against cervical, anal, and other cancers. Polyvalent vaccines target multiple HPV strains including high-risk ones. Higher demand for these vaccines is seen in regions with government-endorsed vaccination programs. With growing awareness of HPV's role in cancer, polyvalent vaccines are preferred for their comprehensive coverage, appealing to healthcare providers and public health programs.The Distribution Channel Segment is Expected to Propel Human Papillomavirus (HPV) Vaccine Market Demand _x000D__x000D_
The market segmentation by distribution channels includes hospital and retail pharmacies, government suppliers, and others. Out of these, hospital and retail pharmacies are expected to dominate the market due to their accessibility and availability of HPV vaccines. Hospitals play a crucial role in vaccination programs, especially for adolescents, while retail pharmacies offer convenient access without appointments.
Human Papillomavirus (HPV) Vaccine Market Analysis by Region
Based on region, the market report covers the United States, EU-4 (Germany, France, Italy, Spain), United Kingdom, Japan, and India.The United States is expected to dominate the market powered by its extensive public health campaigns and government-supported vaccination programs. Polyvalent vaccines are widely used in the region, with broad availability in pharmacies and hospitals, along with insurance coverage contributing to their dominance in the market.
EU-4 and the United Kingdom are also poised to have significant human papillomavirus (HPV) vaccine market share supported by the acceptance of national HPV vaccination and the implementation of school-based vaccination programs. The free HPV vaccines provided by the UK's National Health Service (NHS) are further resulting in elevated vaccination rates among adolescents.
The market in the Japan and India region is rapidly expanding. Japan has seen a resurgence in the HPV vaccine market after public vaccination recommendations were reinstated following a temporary suspension due to safety worries. In India, the government's national immunization campaign, with support from GAVI (The Global Alliance for Vaccines and Immunization), is increasing HPV vaccination coverage despite challenges such as low awareness and limited healthcare access in rural areas.
Leading Players in the Human Papillomavirus (HPV) Vaccine Market
The key features of the market report include patent analysis, grant analysis, clinical trials analysis as well as strategic initiatives including recent partnerships and collaborations by the leading players. The major companies in the market are as follows:Merck & Co., Inc.
Merck & Co., Inc. is an American multinational pharmaceutical company headquartered in Rahway, New Jersey. The company focuses on the discovery, development, manufacturing, and marketing of prescription medicines, biologic therapies, and vaccines.Serum Institute of India Pvt. Ltd.
The Serum Institute of India, based in Pune, is a leading biotechnology company and the largest vaccine manufacturer globally. In partnership with the Indian government, they have developed Cervavac, India's first HPV vaccine. This initiative aims to prevent the deaths of 70,000 women annually from cervical cancer in the country.
Inovio Pharmaceuticals Inc.
Inovio Pharmaceuticals is an American biotech company specializing in synthetic DNA products for cancer and infectious diseases. The FDA granted Breakthrough Therapy Designation in September 2023 for INO -3107, which targets recurrent respiratory papillomatosis, a rare disease caused by HPV-6 and/or HPV-11. Inovio aims to develop DNA medicines for HPV-related conditions, cancer, and infectious diseases to improve patient outcomes.Walvax Biotechnology Co., Ltd
Walvax Biotechnology Co Ltd is a company that develops vaccines and blood products, including the haemophilus influenza vaccine for diseases like meningitis. They also offer drugs for pneumonia, septicemia, cellulitis, arthritis, and epiglottitis. In August 2024, they announced that the World Health Organization has pre-qualified their Walrinvax™ HPV vaccine, showing that it meets WHO standards for safety, efficacy, and quality control, as well as compliance with WHO GMP requirements.Other players in the market are GlaxoSmithKline plc, Johnson & Johnson Services Inc., Bavarian Nordic A/S, Bharat Biotech International Limited (BBIL), Sanofi SA, and Beijing Wantai Biolog Pha Ent Co Ltd.
Key Questions Answered in the Human Papillomavirus (HPV) Vaccine Market Report
- What was the human papillomavirus (HPV) vaccine market value in 2024?
- What is the human papillomavirus (HPV) vaccine market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on type?
- What is the market segmentation based on distribution channels?
- What is the market breakup based on indication?
- What is the market breakup based on the route of administration?
- What are the major factors aiding the human papillomavirus (HPV) vaccine market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the human papillomavirus (HPV) vaccine market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Merck & Co., Inc.
- Serum Institute of India Pvt. Ltd.
- Inovio Pharmaceuticals Inc.
- Walvax Biotechnology Co., Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
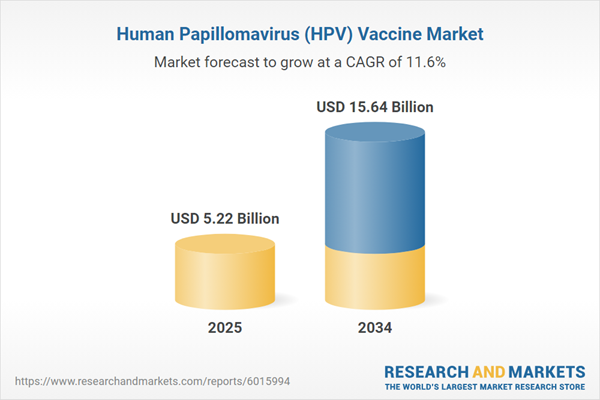
| Estimated Market Value ( USD | $ 5.22 Billion |
| Forecasted Market Value ( USD | $ 15.64 Billion |
| Compound Annual Growth Rate | 11.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |