Global Ischemic Heart Disease (IHD) Drugs Market - Key Trends & Drivers Summarized
Why Are Ischemic Heart Disease (IHD) Drugs Becoming a Critical Focus in Cardiovascular Healthcare?
Ischemic Heart Disease (IHD), also known as coronary artery disease, remains one of the leading causes of morbidity and mortality worldwide, driving a significant demand for effective therapeutic solutions. IHD is characterized by reduced blood supply to the heart muscle, often caused by the buildup of plaque in the coronary arteries, leading to symptoms such as chest pain (angina), shortness of breath, and, in severe cases, heart attacks. The increasing prevalence of IHD, driven by factors such as aging populations, sedentary lifestyles, and the rising incidence of risk factors like hypertension, diabetes, and obesity, has positioned IHD drugs as a critical component of cardiovascular healthcare. As the global burden of IHD continues to grow, there is a heightened focus on developing and optimizing drug therapies that can effectively manage the disease, improve patient outcomes, and reduce the risk of life-threatening cardiac events.The market for IHD drugs is expanding as healthcare systems across the world prioritize cardiovascular health. Key pharmaceutical treatments for IHD include antiplatelet agents, anticoagulants, beta-blockers, calcium channel blockers, nitrates, and lipid-lowering agents. These drugs aim to alleviate symptoms, prevent complications, and enhance the quality of life for patients. In recent years, there has been a surge in research and development activities targeting novel drug classes, such as PCSK9 inhibitors and GLP-1 receptor agonists, which offer additional therapeutic benefits by addressing underlying risk factors like high cholesterol and diabetes. The growing emphasis on personalized medicine is further influencing the IHD drug market, as treatment approaches are increasingly tailored to individual patient profiles based on genetic, biochemical, and lifestyle factors. As a result, the demand for more effective and individualized IHD therapies is driving innovation and growth in the global market.
What Technological and Pharmacological Innovations Are Shaping the Future of IHD Drugs?
Technological and pharmacological advancements are significantly transforming the landscape of IHD drug development, leading to the introduction of more effective and safer treatment options. One of the key innovations is the development of novel drug classes that target specific biological pathways associated with IHD. For instance, PCSK9 inhibitors such as alirocumab and evolocumab have emerged as groundbreaking lipid-lowering agents that significantly reduce LDL cholesterol levels in patients who are unresponsive to traditional statin therapy. By inhibiting the PCSK9 protein, these drugs enhance the liver's ability to remove LDL cholesterol from the bloodstream, thereby lowering the risk of cardiovascular events. Another promising class of drugs is SGLT-2 inhibitors, originally developed for diabetes management, which have demonstrated cardioprotective effects in patients with IHD, reducing the risk of heart failure and myocardial infarction. These pharmacological advancements are broadening the range of therapeutic options available for managing IHD, enabling clinicians to adopt more comprehensive and targeted treatment strategies.Additionally, advancements in drug delivery technologies are enhancing the efficacy and patient compliance of IHD medications. The use of extended-release formulations, transdermal patches, and combination therapies is helping to optimize drug absorption and reduce dosing frequency, thereby improving patient adherence to treatment regimens. Furthermore, the integration of artificial intelligence (AI) and machine learning in drug discovery and development is accelerating the identification of new therapeutic targets and the optimization of clinical trial designs. AI-driven platforms can analyze vast datasets to predict drug efficacy and safety profiles, enabling more efficient drug development processes. The use of pharmacogenomics is another emerging trend, as it allows for the identification of genetic markers that influence drug response, paving the way for personalized therapies tailored to the genetic makeup of individual patients. These technological and pharmacological innovations are not only advancing the field of IHD drug development but are also making treatments more effective, safer, and accessible to a broader patient population.
How Are Market Dynamics and Healthcare Policies Influencing the Ischemic Heart Disease Drugs Market?
The global market for ischemic heart disease drugs is being shaped by a combination of market dynamics and healthcare policies, which vary significantly across regions. The increasing prevalence of IHD, particularly in developed regions like North America and Europe, where aging populations and lifestyle-related risk factors are prevalent, is a major driver of market growth. The demand for IHD drugs is also rising in emerging markets such as Asia-Pacific and Latin America, where economic development, urbanization, and changes in dietary habits are contributing to a growing burden of cardiovascular diseases. In response to these trends, pharmaceutical companies are expanding their presence in these regions, investing in local production facilities, and partnering with regional healthcare providers to improve access to IHD medications.Healthcare policies and reimbursement frameworks are also playing a critical role in shaping the IHD drugs market. In many countries, the cost of IHD drugs is covered by public or private insurance programs, making these therapies more accessible to patients. However, the rising cost of newer therapies, particularly biologics and specialty drugs, has raised concerns about affordability and access. In regions with stringent healthcare budgets, such as Europe, governments and health agencies are implementing cost-containment measures, including price negotiations, reference pricing, and the promotion of generic and biosimilar alternatives. These policies are influencing the pricing strategies of pharmaceutical companies and driving the adoption of cost-effective treatment options. Additionally, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are emphasizing the importance of clinical evidence and real-world data to support the approval and reimbursement of new IHD therapies. This regulatory landscape is pushing companies to conduct more rigorous clinical trials and post-marketing studies, ensuring that new drugs offer meaningful benefits over existing treatments.
The push towards value-based healthcare is another factor impacting the IHD drugs market. Health systems are increasingly focusing on outcomes-based approaches that prioritize treatments demonstrating superior efficacy, safety, and cost-effectiveness. This shift is encouraging pharmaceutical companies to invest in long-term clinical trials and health economics studies to demonstrate the value of their products. Moreover, the growing emphasis on preventive healthcare and risk factor management is shaping the development of IHD therapies that not only treat the disease but also address underlying conditions such as hypertension, diabetes, and high cholesterol. These market dynamics and policy trends are creating both challenges and opportunities for the IHD drugs market, as stakeholders seek to balance innovation with cost containment and access to care.
What Are the Key Growth Drivers Fueling the Expansion of the Ischemic Heart Disease Drugs Market?
The growth in the global ischemic heart disease (IHD) drugs market is driven by several key factors, including the increasing prevalence of cardiovascular diseases, the development of novel drug therapies, and the growing focus on personalized and preventive medicine. One of the primary growth drivers is the rising incidence of IHD, which is closely linked to risk factors such as obesity, diabetes, hypertension, and sedentary lifestyles. The global burden of IHD is expected to continue rising as populations age and lifestyle-related diseases become more prevalent. This growing patient pool is driving the demand for effective IHD therapies that can manage symptoms, prevent complications, and reduce the risk of acute events such as heart attacks. Moreover, the increasing awareness of cardiovascular health and the importance of early intervention are encouraging more individuals to seek medical advice and adhere to prescribed treatments, further boosting the demand for IHD drugs.Another significant growth driver is the ongoing development and commercialization of novel drug therapies that offer new mechanisms of action and improved safety profiles. Recent advances in the understanding of the pathophysiology of IHD have led to the development of innovative drugs that target specific molecular pathways involved in the disease. For instance, the introduction of PCSK9 inhibitors and SGLT-2 inhibitors has expanded the therapeutic landscape for managing IHD, providing options for patients who are not adequately controlled with conventional therapies. The development of combination therapies that target multiple pathways simultaneously is also gaining traction, as these therapies can offer enhanced efficacy and convenience compared to monotherapies. Furthermore, the adoption of precision medicine approaches, which tailor treatments based on individual genetic and clinical profiles, is opening new avenues for targeted IHD therapies that address the unique needs of each patient.
Lastly, the increasing emphasis on preventive healthcare and risk factor management is contributing to the growth of the IHD drugs market. Governments and healthcare organizations are implementing public health initiatives aimed at reducing the incidence of cardiovascular diseases through lifestyle modifications, early diagnosis, and effective management of risk factors such as high cholesterol and hypertension. This preventive approach is driving the use of lipid-lowering agents, antihypertensives, and other cardioprotective drugs as part of comprehensive cardiovascular care strategies. Additionally, the integration of digital health technologies, such as wearable devices and mobile health applications, is enabling continuous monitoring of cardiovascular health and facilitating better patient adherence to treatment plans. These technological advancements are enhancing the management of IHD, reducing the risk of complications, and improving patient outcomes. As the global focus on cardiovascular health intensifies and as new therapies continue to emerge, the IHD drugs market is poised for sustained growth, driven by innovation, increased awareness, and the need for more effective and personalized treatment options.
Report Scope
The report analyzes the Ischemic Heart Disease (IHD) Drugs market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Disease Class (Angina Pectoris, Myocardial Infarction).
- Geographic Regions/Countries:World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Angina Pectoris Drugs segment, which is expected to reach US$4.3 Billion by 2030 with a CAGR of a 2.5%. The Myocardial Infarction Drugs segment is also set to grow at 1.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.7 Billion in 2024, and China, forecasted to grow at an impressive 4.7% CAGR to reach $1.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ischemic Heart Disease (IHD) Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ischemic Heart Disease (IHD) Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ischemic Heart Disease (IHD) Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amgen, Inc., AstraZeneca Plc, Bayer AG, Biocon Ltd., Camber Pharmaceuticals, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 56 companies featured in this Ischemic Heart Disease (IHD) Drugs market report include:
- Amgen, Inc.
- AstraZeneca Plc
- Bayer AG
- Biocon Ltd.
- Camber Pharmaceuticals, Inc.
- Covis Pharma
- Dr. Reddy's Laboratories Ltd.
- Kowa Pharmaceuticals America, Inc.
- Lupin Ltd.
- Medicure, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amgen, Inc.
- AstraZeneca Plc
- Bayer AG
- Biocon Ltd.
- Camber Pharmaceuticals, Inc.
- Covis Pharma
- Dr. Reddy's Laboratories Ltd.
- Kowa Pharmaceuticals America, Inc.
- Lupin Ltd.
- Medicure, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
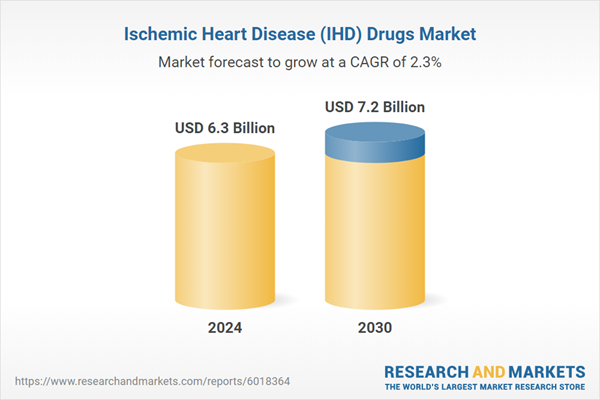
| Estimated Market Value ( USD | $ 6.3 Billion |
| Forecasted Market Value ( USD | $ 7.2 Billion |
| Compound Annual Growth Rate | 2.3% |
| Regions Covered | Global |