The growing prevalence of chronic disorders is a major concern in the U.S., with diseases such as diabetes, cardiovascular conditions, respiratory disorders, and neurological diseases such as Alzheimer's and Parkinson's becoming increasingly common. For instance, according to the American Diabetes Association, in 2021, 38.4 million Americans, or 11.6% of the population, were living with diabetes. Among them, 2 million individuals have type 1 diabetes, including approximately 304,000 children and adolescents. These conditions place a significant strain on healthcare systems and demand advanced diagnostic, therapeutic, and monitoring solutions. As the population ages and lifestyle factors contribute to the rise of these chronic illnesses, the need for effective management and treatment options continues to grow, driving the demand for electro-medical and electrotherapeutic devices.
Technological advancements are expected to significantly boost the growth of the U.S. electro-medical and electrotherapeutic apparatus market as companies continue to innovate and introduce new, advanced products. Market players are increasingly focused on developing advanced devices that address the evolving healthcare needs of patients and healthcare providers. A key aspect of this innovation is the integration of advanced technologies such as artificial intelligence, wearable devices, and continuous monitoring systems into medical equipment. As a result, companies are seeking regulatory approvals from government bodies, such as the U.S. Food and Drug Administration (FDA), to bring these new technologies to market. In June 2024, Abbott announced that the FDA had cleared two new over-the-counter continuous glucose monitoring (CGM) systems - Lingo and Libre Rio. Both products are built on Abbott's well-established FreeStyle Libre CGM technology, which is already used by around 6 million people globally. These innovations reflect the ongoing efforts of companies to enhance healthcare solutions and meet the growing demand for advanced, accessible diagnostic tools.
U.S. Electro-medical And Electrotherapeutic Apparatus Market Highlights
- Diagnostic equipment segment dominated the product segment by capturing a share of 38.2% in 2024.
- The therapeutic equipment segment is anticipated to grow at the fastest CAGR during the forecast period.
- On the basis of application cardiology segment held the largest market share by capturing a share of 28.3% in 2024.
- Neurology segment is expected to grow at a fastest rate during the forecast period.
- Among end use, hospitals held a majority share of the market of 45.8% in 2024.
- Diagnostic centers segment is expected to grow at a fastest rate over the forecast period.
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
Table of Contents
Companies Mentioned
- GE HealthCare
- Koninklijke Philips N.V.
- Siemens Healthineers AG
- Medtronic
- Abbott
- Boston Scientific Corporation
- Fujifilm Holdings Corporation
- Zimmer Biomet
- Nihon Kohden Corporation
- OMRON Healthcare, Inc.
- Invacare Corporation
Table Information
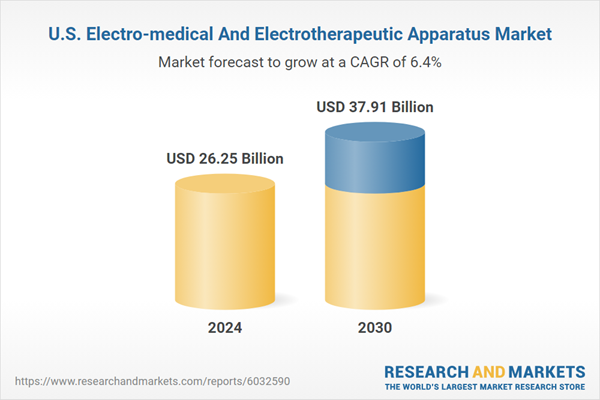
| Report Attribute | Details |
|---|---|
| No. of Pages | 90 |
| Published | November 2024 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 26.25 Billion |
| Forecasted Market Value ( USD | $ 37.91 Billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | United States |
| No. of Companies Mentioned | 11 |