Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The Impact of the COVID-19 Pandemic
The COVID-19 pandemic, a global health crisis of unprecedented scale, has not only challenged healthcare systems and economies but has also reshaped the landscape of medical diagnostics. One of the most significant outcomes of this crisis has been the exponential growth of the Spain Rapid Test Kit Market. These diagnostic tools have played a pivotal role in controlling the spread of the virus, and their influence is poised to extend beyond the pandemic.COVID-19 presented an urgent need for mass testing to identify infected individuals quickly. Traditional laboratory-based testing methods were often time-consuming, and the demand for faster results led to the widespread adoption of rapid test kits. These kits allowed for on-the-spot testing, facilitating immediate isolation and contact tracing measures. As a result, the market for rapid test kits witnessed unprecedented growth, with healthcare facilities, governments, and organizations investing heavily in these diagnostic tools.
Spain was one of the hardest-hit countries in Europe during the initial waves of COVID-19, with over 13 million reported cases as of late 2024. This resulted in a substantial demand for testing kits, including PCR tests and rapid antigen tests, to track and control the virus's spread.
The pandemic underscored the importance of readily accessible testing. Rapid test kits are user-friendly and do not require specialized equipment or trained professionals, making them suitable for use in various settings. Beyond hospitals and clinics, they found applications in schools, workplaces, airports, and even in the homes of individuals, enabling a broader range of users to access testing services. This ease of use expanded the market's reach and accessibility.
As mass vaccination campaigns rolled out, rapid test kits played a complementary role. They were used to verify the vaccination status of individuals and screen for breakthrough cases, helping to ensure public safety. This ongoing need for testing, even as vaccination efforts progress, is expected to sustain the demand for rapid test kits well into the future.
The emergence of new COVID-19 variants and the potential for future pandemics have highlighted the importance of preparedness. Rapid test kits are versatile tools that can be quickly adapted to detect new variants or other infectious diseases. As a result, investments in research and development in this sector have surged, fostering innovation and creating a robust ecosystem for the continuous improvement of diagnostic technologies.
The pandemic has heightened awareness about health and disease prevention. People are more proactive about monitoring their health and taking precautionary measures. Rapid test kits provide a means for individuals to perform regular health checks and take immediate action if necessary. This trend in health consciousness is likely to contribute to the sustained growth of the rapid test kit market.
Increasing Health Awareness
In recent years, Spain has witnessed a remarkable surge in health awareness, with individuals taking a proactive approach to their well-being. This growing awareness has had a profound impact on the healthcare landscape, particularly in the utilization of rapid test kits. These versatile and user-friendly diagnostic tools are experiencing an upswing in demand.One of the most significant factors driving the growth of the Spain Rapid Test Kit Market is the increasing sense of personal responsibility for health. Individuals are now more inclined to take charge of their well-being by monitoring their health regularly. Rapid test kits empower people to perform quick, reliable, and non-invasive health checks from the comfort of their own homes.
Health awareness is closely linked to accessibility and convenience. People want quick and hassle-free solutions to monitor their health. Rapid test kits offer just that. They are designed to be user-friendly, requiring no specialized training or equipment. This ease of use enables individuals to conduct tests independently, making health monitoring more accessible than ever before.
As health consciousness grows, people are increasingly focused on disease prevention rather than just treating illnesses. Rapid test kits help individuals detect potential health issues at an early stage, allowing for timely intervention and lifestyle adjustments. The ability to catch health concerns before they become serious conditions is a compelling driver of the market's growth.
Rapid test kits cater to a wide range of health needs, extending their relevance beyond infectious disease testing. They can be employed for pregnancy tests, drug screening, cardiac marker analysis, diabetes management, and more. The versatility of these kits means they can address various aspects of health and well-being, making them indispensable tools in a world where holistic health is a priority.
The recent challenges posed by the COVID-19 pandemic have emphasized the importance of preparedness and the need for accessible and rapid diagnostic tools. Public health initiatives have been reinvigorated, contributing to increased health awareness and a growing demand for rapid test kits.
Accessibility and Convenience
In today's fast-paced world, accessibility and convenience are key drivers of consumer choices, and the healthcare sector is no exception. The Spain Rapid Test Kit Market has experienced significant growth, thanks to the ever-increasing emphasis on making healthcare solutions more accessible and user-friendly.One of the primary factors contributing to the growth of the Spain Rapid Test Kit Market is the user-friendly design of these diagnostic tools. These kits are designed for ease of use, with clear instructions that enable individuals to perform tests without the need for specialized training or professional assistance. This simplicity is especially important for self-administered tests, as it empowers consumers to take control of their health.
Accessibility and convenience go hand in hand with the trend of home testing. Rapid test kits have played a significant role in this revolution. They allow people to conduct health assessments in the comfort of their own homes, eliminating the need for travel and appointments with healthcare professionals. This has become increasingly important, especially in a world where time and convenience are at a premium.
The Spain Rapid Test Kit Market's growth is closely tied to the potential cost savings for both individuals and healthcare systems. By providing easy access to diagnostic information, these kits can help reduce the need for frequent visits to healthcare facilities, which can be costly and time-consuming. Additionally, early detection through home testing can lead to more effective and less costly interventions.
Rapid test kits have democratized healthcare, making it accessible to a broader segment of the population. Those in remote or underserved areas can now access diagnostic services easily. This has the potential to reduce health disparities and ensure that everyone, regardless of their location or socioeconomic status, has access to crucial health information.
Beyond infectious disease testing, rapid test kits are increasingly used for monitoring chronic health conditions such as diabetes and heart disease. Patients can regularly track their health markers and make informed decisions about their treatment or lifestyle. This proactive approach to healthcare management is possible due to the accessibility and convenience of these tests.
Key Market Challenges
Accuracy and Reliability
One of the foremost challenges facing the rapid test kit market is ensuring consistent accuracy and reliability. While these kits are known for their speed and convenience, there can be variations in their performance. False positives and negatives can lead to inaccurate diagnoses, potentially impacting patient care and public health measures. Ensuring that the tests consistently produce accurate results, especially in critical applications like disease detection, is crucial. Inaccurate results could lead to misdiagnosis, delayed treatments, or unnecessary interventions.One aspect of accuracy is ensuring high sensitivity (the ability to correctly identify those with the condition) and specificity (the ability to correctly identify those without the condition). Low sensitivity can lead to false negatives, where a person with the condition is incorrectly identified as healthy. Low specificity, on the other hand, can lead to false positives, where a healthy person is mistakenly diagnosed with a condition.
Quality Control
Quality control is a critical challenge in the development and deployment of rapid test kits. For these kits to be reliable, they must meet stringent quality standards in terms of performance and consistency across all batches produced. The process of producing test kits involves complex steps, and any deviation in manufacturing can lead to variations in the quality of the final product. This includes issues with raw materials, reagent quality, or improper calibration of the kits.These inconsistencies can compromise the test’s ability to consistently provide accurate results, which is especially concerning for healthcare professionals who rely on these kits for timely decisions. Another challenge is ensuring proper storage and transportation conditions for the test kits. Some kits, such as those that rely on biological materials or certain chemical reagents, may be sensitive to temperature or humidity. Improper storage or transport - such as prolonged exposure to heat or freezing - can degrade the test kit’s performance, leading to compromised results.
False Positives and Negatives
False positives and false negatives are among the most significant risks in the rapid test kit market. These results can have serious consequences, including incorrect treatment decisions, delayed diagnoses, and public health implications. A false positive occurs when a test incorrectly identifies a person as having a disease or condition when they do not. This can result in unnecessary treatment, emotional distress, and increased healthcare costs. For instance, in the case of infectious diseases, false positives can lead to unnecessary quarantines or the use of resources for people who do not require them. In chronic conditions such as cancer, a false positive might result in unnecessary invasive procedures or treatments.Key Market Trends
Multiplex Testing
Multiplex testing is a growing trend in the rapid test kit market that allows for the simultaneous detection of multiple pathogens or biomarkers from a single sample. This advancement is especially valuable in settings where rapid diagnosis is critical, such as in infectious disease outbreaks, emergency care, and point-of-care environments. Traditional diagnostic methods often require multiple tests for detecting different pathogens, which can be time-consuming and resource-intensive. Multiplex testing streamlines this process by enabling the detection of several pathogens at once, thus providing quicker results and reducing the time spent on diagnosing multiple conditions. For example, a single multiplex test can detect various respiratory viruses (such as influenza, COVID-19, and RSV) in a single sample, which is crucial during flu seasons or pandemics.Targeted Pathogen Detection
Targeted pathogen detection is another key trend in the rapid test kit market, focusing on identifying specific pathogens with high precision. Unlike traditional broad-spectrum tests, targeted pathogen detection aims to pinpoint particular organisms or diseases, enhancing the accuracy and relevance of the diagnostic results. Targeted detection technologies, such as PCR (Polymerase Chain Reaction), CRISPR-based diagnostics, and immunoassays, are designed to detect specific genetic material, proteins, or antigens associated with particular pathogens. This approach offers greater sensitivity and specificity, reducing the chances of false positives or negatives and improving the overall reliability of the test results.Data Integration and Connectivity
As the healthcare sector increasingly moves toward digital solutions, data integration and connectivity are becoming key components in the evolution of rapid test kits. These technologies allow test results to be seamlessly integrated into broader healthcare networks, improving patient management and ensuring more efficient use of resources. Modern rapid test kits can now be connected to electronic health record (EHR) systems, laboratory information management systems (LIMS), or cloud-based platforms. This integration enables real-time sharing of test results between healthcare providers, allowing for faster decision-making and coordinated care, especially in multi-site healthcare facilities or during disease outbreaks.Segmental Insights
Type Insights
Based on Type, the Rapid Antigen Test is set to dominate the Spain Rapid Test Kit Market for several compelling reasons. First and foremost, its simplicity and speed make it a highly preferred choice for quick and efficient COVID-19 detection. With results typically available within minutes, it offers an invaluable tool for fast and decisive decision-making in various settings, from healthcare facilities to border checkpoints and workplaces. Additionally, its cost-effectiveness and ease of use have made it accessible to a wide range of users, including individuals and organizations. Rapid Antigen Tests have proven their reliability in identifying active COVID-19 cases, further solidifying their position as a go-to solution in the ongoing battle against the pandemic. As Spain and the world continue to manage the impact of COVID-19, the Rapid Antigen Test's convenience, affordability, and accuracy place it at the forefront of the Spain Rapid Test Kit Market, ensuring its dominance in the coming years.Regional Insights
Central Region North Spain is poised to dominate the Spain Rapid Test Kit Market for several compelling reasons. First and foremost, the region boasts a strong infrastructure for research and development, facilitating the rapid innovation and production of cutting-edge test kits. Likewise, its strategic location as a central hub for transportation and distribution enables efficient market access and nationwide coverage.Additionally, the presence of a skilled workforce, combined with a robust healthcare ecosystem, ensures the highest quality standards and regulatory compliance. Central Region North Spain's commitment to fostering collaboration between industry and academia has fueled a culture of innovation, positioning it as a key player in the rapidly evolving diagnostic industry. All these factors collectively make Central Region North Spain the frontrunner in the Spain Rapid Test Kit Market, offering a competitive edge and promising growth prospects in this critical healthcare sector.
Key Market Players
- Abbott Laboratories Inc
- Danaher Corporation
- Becton Dickinson, S.A.
- PerkinElmer, Inc
- Siemens Healthineers España
- Thermo Fischer Scientific, Inc.
- Bio-Rad Laboratories, Inc.
- bioMérieux España
- Cellex, Inc.
- Diasorin Iberia S.A.
- Eurofins Scientific SE
Report Scope:
In this report, the Spain Rapid Test Kit Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Spain Rapid Test Kit Market, By Type:
- Rapid Antigen Test
- Rapid Antibody Test
Spain Rapid Test Kit Market, By Product Type:
- Over-the-counter {OTC} Rapid Test Kit
- Professional Rapid Test Kit
Spain Rapid Test Kit Market, By Technology:
- Lateral Flow Assays
- Solid Phase
- Agglutination
- Immunospot Assay
Spain Rapid Test Kit Market, By Duration:
- Less than 30 Minutes
- Less than 10 Minutes
- Less than 1 Hour
- 1 Hour - 2 Hour
- Others
Spain Rapid Test Kit Market, By Application:
- Infectious Disease
- Glucose Monitoring
- Pregnancy & Fertility
- Toxicology
- Cardiology
- Oncology
- Others
Spain Rapid Test Kit Market, By End User:
- Hospitals & Clinics
- Home Care
- Diagnostic Centers
- Others
Spain Rapid Test Kit Market, By Region:
- Central Region North Spain
- Aragon & Catalonia
- Andalusia, Murcia & Valencia
- Madrid, Extremadura & Castilla
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Spain Rapid Test Kit Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories Inc
- Danaher Corporation
- Becton Dickinson, S.A.
- PerkinElmer, Inc
- Siemens Healthineers España
- Thermo Fischer Scientific, Inc.
- Bio-Rad Laboratories, Inc.
- bioMérieux España
- Cellex, Inc.
- Diasorin Iberia S.A.
- Eurofins Scientific SE
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | December 2024 |
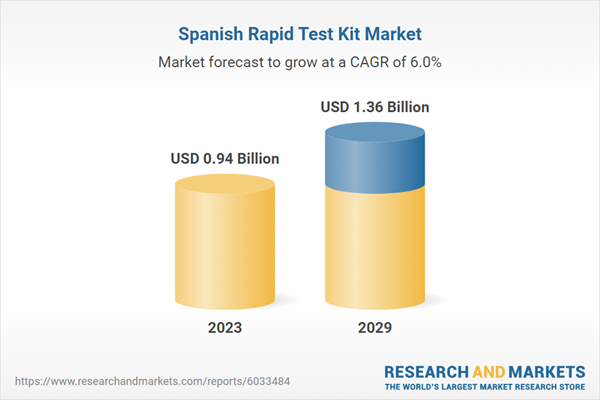
| Forecast Period | 2023 - 2029 |
| Estimated Market Value ( USD | $ 0.94 Billion |
| Forecasted Market Value ( USD | $ 1.36 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Spain |
| No. of Companies Mentioned | 11 |