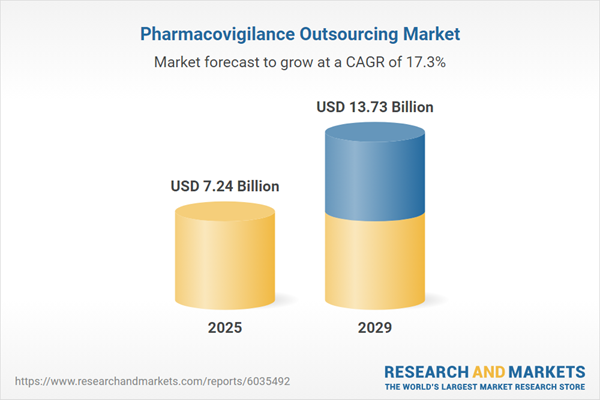
The pharmacovigilance outsourcing market size has grown rapidly in recent years. It will grow from $6.16 billion in 2024 to $7.24 billion in 2025 at a compound annual growth rate (CAGR) of 17.6%. The growth in the historic period can be attributed to increasing drug development activities, stringent regulatory requirements, a rising incidence of adverse drug reactions, growing patient safety concerns, and increasing outsourcing by small and mid-sized companies.
The pharmacovigilance outsourcing market size is expected to see rapid growth in the next few years. It will grow to $13.73 billion in 2029 at a compound annual growth rate (CAGR) of 17.3%. The growth in the forecast period can be attributed to the growth of biologics and biosimilars, the rising focus on personalized medicine, the rising demand for real-world evidence, the increasing complexity of drug safety profiles, and the growing emphasis on patient-centric care. Major trends in the forecast period include the increasing use of big data analytics, a rise in partnerships and collaborations, the adoption of cloud-based pharmacovigilance solutions, the integration of pharmacovigilance with digital health platforms, and the development of specialized pharmacovigilance services for gene therapies.
The growth of the pharmacovigilance outsourcing market is anticipated to be driven by the increasing number of clinical trials. Clinical trials are research studies designed to assess the safety, effectiveness, and potential side effects of new treatments, drugs, or medical devices. The rise in clinical trials is fueled by factors such as the emphasis on precision medicine, the growing prevalence of chronic diseases, regulatory support, and the globalization of research. Outsourcing pharmacovigilance in clinical trials improves drug safety monitoring by utilizing specialized expertise and resources to ensure regulatory compliance and enhance efficiency. For instance, in November 2023, the Association of the British Pharmaceutical Industry reported a slight increase of 4.3% in the number of industry-sponsored clinical trials in the UK, rising from 394 in 2021 to 411 in 2022. Thus, the growing number of clinical trials is driving the expansion of the pharmacovigilance outsourcing market.
Key players in the pharmacovigilance outsourcing market are focusing on developing innovative solutions, such as cloud-based data lake platforms, to optimize pharmacovigilance case processing and safety data management. These platforms help enhance clinical research registries and ensure regulatory compliance. A cloud-based data lake platform is a centralized repository that stores and manages large volumes of raw data in its native format until needed. For example, in December 2023, Thermo Fisher Scientific Inc., a U.S.-based biotechnology and laboratory equipment company, introduced CorEvidence. This platform is designed to improve clinical research registries by streamlining pharmacovigilance case processing and managing multiple data sources. It optimizes the coding, classification, and reporting of adverse events, offering scalable, compliant, and auditable safety management while supporting long-term post-authorization safety studies across various therapeutic areas.
In November 2023, Permira Advisers LLP, a UK-based investment firm, acquired Ergomed PLC for $0.904 billion. This acquisition is intended to support Ergomed's growth by leveraging Permira’s expertise, capital, and global network to enhance Ergomed’s commercial, technological, and geographic capabilities and facilitate strategic acquisitions in the contract research organization (CRO) and pharmacovigilance (PV) sectors. Ergomed PLC, based in the UK, offers drug development services, including pharmacovigilance.
Major companies operating in the pharmacovigilance outsourcing market are ACCENTURE PLC, International Business Machines Corporation (IBM), Tata Consultancy Services, Capgemini SE, Cognizant Technology Solutions Corporation, IQVIA Holdings Inc., Laboratory Corporation of America Holdings (Labcorp), Wipro Limited, ICON PLC, Syneos Health, Genpact limited, Parexel International Corp, Veeva Systems Inc., Medpace Holdings Inc., ProPharma Group, Pharmalex GmbH, PrimeVigilance Ltd., Global Pharma Tek, Veristat LLC, United BioSource LLC, Lambda Therapeutic Research, ArisGlobal, Biomapas.
North America was the largest region in the pharmacovigilance outsourcing market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the pharmacovigilance outsourcing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the pharmacovigilance outsourcing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Pharmacovigilance outsourcing involves hiring external service providers to manage and perform pharmacovigilance activities for pharmaceutical companies. These activities encompass detecting, assessing, understanding, and preventing adverse effects or other drug-related issues.
The primary types of pharmacovigilance outsourcing include adverse drug reaction capture (ADR), case processing, reporting and submission, report publishing, quality checks, risk management, knowledge management, and enabling architecture. ADR capture involves identifying, documenting, and analyzing harmful or unintended reactions to medications to ensure drug safety and enhance patient care. These services are typically offered by contract research organizations and business process outsourcing companies. The main users of these outsourced services include the pharmaceutical industry, research organizations, and other stakeholders.
The pharmacovigilance outsourcing market research report is one of a series of new reports that provides pharmacovigilance outsourcing market statistics, including pharmacovigilance outsourcing industry global market size, regional shares, competitors with a pharmacovigilance outsourcing market share, detailed pharmacovigilance outsourcing market segments, market trends and opportunities, and any further data you may need to thrive in the pharmacovigilance outsourcing industry. This pharmacovigilance outsourcing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The pharmacovigilance outsourcing market includes revenues earned by entities by providing services such as pharmacovigilance database management, assessment services, medical information call center services, regulatory consulting and strategy services, pharmacovigilance compliance and auditing, and aggregate reporting and benefit-risk evaluation. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Pharmacovigilance Outsourcing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pharmacovigilance outsourcing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pharmacovigilance outsourcing ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The pharmacovigilance outsourcing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Adverse Drug Reaction Capture (ADR); Case Processing; Reporting and Submission; Report Publishing; Quality Check; Risk Management; Knowledge Management; Enabling Architecture2) By Service Provider: Contract Research Organization; Business Processing Outsourcing
3) By End User: Pharmaceutical Industry; Research Organization; Others End Users
Subsegments:
1) Adverse Drug Reaction Capture (ADR): Automated ADR Reporting Tools; Manual ADR Reporting Solutions; ADR Data Mining Software2) Case Processing: Case Intake and Triage Services; Medical Review and Evaluation Services; Data Entry and Management Services
3) Reporting and Submission: Regulatory Reporting Services; Submission to Health Authorities; Compliance Tracking Services
4) Report Publishing: Clinical Study Report Publishing; Safety Update Report Publishing; Final Study Report Publishing
5) Quality Check: Quality Assurance Services; Audit and Inspection Services; Compliance Monitoring Services
6) Risk Management: Risk Assessment Services; Risk Minimization Strategies; Benefit-Risk Evaluation Services
7) Knowledge Management: Data Management Solutions; Pharmacovigilance Database Management; Knowledge Sharing Platforms
8) Enabling Architecture: IT Infrastructure Solutions; Data Integration Services; Technology Platform Support
Key Companies Mentioned: ACCENTURE PLC; International Business Machines Corporation (IBM); Tata Consultancy Services; Capgemini SE; Cognizant Technology Solutions Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Pharmacovigilance Outsourcing market report include:- ACCENTURE PLC
- International Business Machines Corporation (IBM)
- Tata Consultancy Services
- Capgemini SE
- Cognizant Technology Solutions Corporation
- IQVIA Holdings Inc.
- Laboratory Corporation of America Holdings (Labcorp)
- Wipro Limited
- ICON PLC
- Syneos Health
- Genpact limited
- Parexel International Corp
- Veeva Systems Inc.
- Medpace Holdings Inc.
- ProPharma Group
- Pharmalex GmbH
- PrimeVigilance Ltd.
- Global Pharma Tek
- Veristat LLC
- United BioSource LLC
- Lambda Therapeutic Research
- ArisGlobal
- Biomapas
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 7.24 Billion |
| Forecasted Market Value ( USD | $ 13.73 Billion |
| Compound Annual Growth Rate | 17.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |