The China market dominated the Asia Pacific Life Sciences Quality Management Software Market by country in 2023, and is expected to continue to be a dominant market till 2031; thereby, achieving a market value of $548.5 million by 2031. The Japan market is registering a CAGR of 12.6% during 2024-2031. Additionally, the India market is expected to showcase a CAGR of 14% during 2024-2031.
The adoption of this software has been accelerating due to several factors, including increased regulatory scrutiny, technological advancements, and the need for improved operational efficiency. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Organization for Standardization (ISO) have stringent requirements for product quality and safety, driving the need for more robust quality management systems.
The life sciences industry is subject to stringent regulatory requirements, which vary across regions and product categories. Failing to comply with these regulations can lead to significant financial penalties, reputational damage, and even product recalls. Life sciences QMS software helps organizations meet all regulatory requirements by automating compliance processes, maintaining accurate records, and providing real-time reporting capabilities.
The life sciences quality management software market is experiencing robust growth, driven by the continuous expansion of the pharmaceutical and biotechnology sectors in key regions like China and India. Life sciences QMS solutions are crucial in India to manage complex research and development processes, clinical trials, and manufacturing compliance. As Indian biotech companies grow in scale and scope, the demand for advanced software solutions that ensure regulatory compliance, operational efficiency, and product safety will rise, making the country a key market. Thus, with their growing life sciences sectors, both nations are expected to see continued investments in advanced technologies like QMS to enhance quality control, reduce operational risks, and comply with international standards.
List of Key Companies Profiled
- AmpleLogic
- Hexagon AB
- Qualityze Inc.
- Veeva Systems, Inc.
- SimplerQMS
- Dassault Systemes SE
- MasterControl Solutions Inc.
- Ideagen Plc
- IQVIA Holdings, Inc.
- Honeywell International Inc.
Market Report Segmentation
By Application- Data Management
- Regulatory & Compliance Management
- Corrective Action Preventive Action (CAPA) Management
- Change Management
- Audit Management
- Risk Management
- Non-Conformances Management
- Supplier Management
- Training Management
- Inspection Management
- Other Application
- Cloud & Web-based
- On-premises
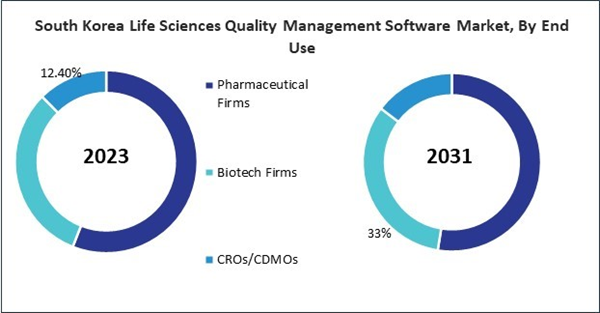
- Pharmaceutical Firms
- Biotech Firms
- CROs/CDMOs
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
Table of Contents
Companies Mentioned
- AmpleLogic
- Hexagon AB
- Qualityze Inc.
- Veeva Systems, Inc.
- SimplerQMS
- Dassault Systemes SE
- MasterControl Solutions Inc.
- Ideagen Plc
- IQVIA Holdings, Inc.
- Honeywell International Inc.