Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Technological Advancements in NIPT
Advancements in technology have significantly contributed to the growth of the NIPT market in Australia. The development of highly accurate, fast, and non-invasive genetic testing methods such as cell-free fetal DNA testing has boosted the adoption of NIPT. These tests provide a safer alternative to traditional invasive procedures like amniocentesis or chorionic villus sampling (CVS), which carry a risk of miscarriage. NIPT offers the ability to screen for a range of chromosomal conditions such as Down syndrome, Edwards syndrome, and Patau syndrome, without the need for invasive techniques.As a result, healthcare providers and expectant parents are increasingly opting for NIPT due to its non-invasive nature, higher accuracy, and reduced risk. Furthermore, the expansion of digital health solutions and mobile applications for monitoring pregnancies has also fueled the growth of the NIPT market, enabling greater access to genetic testing. This, coupled with a more informed population of prospective parents, has led to increased demand for NIPT in Australia.
Government Support and Reimbursement Policies
The Australian government’s policies, including Medicare’s reimbursement of NIPT for high-risk pregnancies, have been a significant driver in the growth of the market. With the inclusion of NIPT as a subsidized test for high-risk groups, including women over the age of 35, those with a family history of genetic conditions, or those with abnormal ultrasound findings, there is increased accessibility to this advanced screening option. Reimbursement policies make NIPT more affordable and accessible for a larger segment of the population. Additionally, public health initiatives have aimed at raising awareness of prenatal genetic testing, making it a standard part of prenatal care in Australia. This has not only encouraged wider acceptance among expectant mothers but has also provided greater confidence to healthcare professionals in recommending NIPT as a first-line screening tool, further driving its adoption in clinical practice.Key Market Challenges
Ethical and Social Concerns
One of the major challenges to the growth of the NIPT market in Australia is the ethical and social implications of genetic testing. NIPT raises concerns regarding the potential for selective abortion based on genetic findings, leading to debates surrounding reproductive rights and ethics. While NIPT offers valuable information about the fetus's health, its ability to detect even minor genetic anomalies can result in tough decisions for parents, especially when it comes to conditions that may not severely affect the quality of life.There are concerns that widespread availability of genetic testing may contribute to societal pressures to conform to certain genetic norms. Additionally, the emotional and psychological impact of receiving information about fetal conditions that may not be treatable or preventable remains a key challenge in terms of counseling and support for expectant families. Addressing these concerns through counseling and offering ethical guidance remains a vital component for the responsible use of NIPT.
Regulatory and Legal Barriers
The regulatory landscape surrounding NIPT in Australia presents another challenge. Despite the growth of the market, there is still uncertainty regarding the legal framework and regulation of genetic testing, particularly as it relates to privacy, data protection, and the handling of sensitive genetic information. NIPT involves the collection of DNA from both the mother and the fetus, raising questions about data security and confidentiality. Additionally, as the technology evolves, there is a need for consistent regulation across both private and public healthcare sectors. Without robust regulatory guidelines, there may be variability in test accuracy and standards of practice, which can undermine trust in the technology. Moreover, legal issues related to informed consent, the accessibility of genetic counseling services, and the potential misuse of genetic data for purposes other than prenatal screening pose significant challenges for the future of the NIPT market in Australia.Key Market Trends
Increased Uptake of NIPT in Low-Risk Pregnancies
Initially, NIPT was primarily targeted at women with high-risk pregnancies due to factors such as maternal age or family history of genetic disorders. However, there has been a notable trend towards the increased adoption of NIPT for low-risk pregnancies. With advancements in technology and reduced costs, NIPT has become more accessible to a broader population.Many expectant mothers, even those without risk factors, now opt for NIPT as a preferred choice for early screening, providing peace of mind and more accurate results compared to traditional screening methods such as the combined first-trimester screening. This shift is also due to increasing awareness of the benefits of NIPT, such as its non-invasive nature, higher accuracy, and the ability to detect a wider range of conditions. As a result, private healthcare providers have expanded their offerings to include NIPT for low-risk patients, leading to a surge in demand for these tests.
Integration of Artificial Intelligence and Machine Learning in NIPT
The incorporation of artificial intelligence (AI) and machine learning (ML) into the NIPT process is a significant trend shaping the Australian market. AI and ML algorithms are being increasingly utilized to enhance the accuracy and efficiency of genetic analysis by interpreting large datasets more effectively. These technologies help refine test results, reduce the occurrence of false positives or negatives, and allow for more precise risk assessments of chromosomal abnormalities. In addition, AI can assist in the early detection of other health conditions beyond the typical chromosomal anomalies, such as metabolic or developmental disorders. The integration of AI is also improving the speed of results, allowing for quicker decision-making by healthcare professionals and providing faster reassurance for parents. As these technologies continue to advance, NIPT is expected to evolve, providing even more personalized and comprehensive prenatal care options for Australian expectant mothers.Segmental Insights
Technology Insights
Based on the technology, the Next-generation sequencing category have emerged as the dominant segment. NGS enables comprehensive and highly accurate genomic analysis by sequencing large amounts of DNA at once. This technology has revolutionized prenatal screening by offering non-invasive, highly sensitive, and accurate detection of chromosomal conditions, such as Down syndrome, Edwards syndrome, and Patau syndrome.With NGS, fetal DNA can be detected in the maternal bloodstream, and the risk of genetic disorders can be assessed with exceptional precision without the need for invasive procedures like amniocentesis or CVS, which carry a risk of miscarriage. The growing reliance on NGS in NIPT reflects the technology’s ability to provide comprehensive results, with reduced false positive and false negative rates compared to older technologies. NGS also allows for a broader range of genetic conditions to be tested for, including microdeletions, further expanding its utility. As the cost of sequencing continues to fall and the technology improves, NGS is expected to continue dominating the NIPT landscape in Australia, leading to more widespread adoption of these tests across a broader population, including low-risk pregnancies.
Regional Insights
Victoria and Tasmania are leading regions in the Australian Non-Invasive Prenatal Testing (NIPT) market, primarily due to their well-established healthcare infrastructure and increasing awareness of advanced prenatal screening. These regions have seen higher adoption rates of NIPT, driven by the availability of specialized healthcare facilities and the widespread use of NIPT as part of routine prenatal care. Victoria, with its major metropolitan centers like Melbourne, is home to many private and public hospitals that offer advanced testing, including NIPT using next-generation sequencing (NGS).Tasmania, with its more concentrated population and healthcare network, has also seen a rise in NIPT uptake, especially as healthcare providers continue to promote the benefits of non-invasive testing for expectant mothers. Both regions are witnessing greater access to genetic counseling services, which, combined with strong government health policies, have encouraged women to opt for NIPT, particularly in urban centers. As more expectant mothers in these regions recognize the advantages of NIPT in terms of its accuracy, safety, and convenience, the demand for these services continues to grow, further establishing Victoria and Tasmania as dominant markets within the broader Australian landscape.
Key Market Players
- F. Hoffmann-La Roche Ltd
- Virtus Health
- Illumina, Inc.
- BGI
- Natera, Inc.
- Monash IVF Group
Report Scope:
In this report, the Australia Non-Invasive Prenatal Testing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Australia Non-Invasive Prenatal Testing Market, By Technology:
- Next-generation sequencing
- Polymerase chain reaction
- Others
Australia Non-Invasive Prenatal Testing Market, By Application:
- Trisomy Detection
- Microdeletion Detection
- Sex Chromosome
- Aneuploidy Detection
- Others
Australia Non-Invasive Prenatal Testing Market, By End Use:
- Hospital and Clinics
- Diagnostic Laboratories
- Others
Australia Non-Invasive Prenatal Testing Market, By Region:
- Victoria & Tasmania
- Queensland
- Western Australia
- Northern Territory & Southern Australia
- Australia Capital Territory & New South Wale
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Australia Non-Invasive Prenatal Testing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd
- Virtus Health
- Illumina, Inc.
- BGI
- Natera, Inc.
- Monash IVF Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 90 |
| Published | January 2025 |
| Forecast Period | 2024 - 2030 |
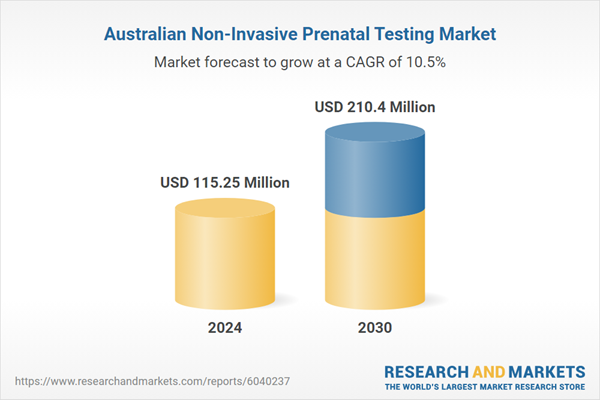
| Estimated Market Value ( USD | $ 115.25 Million |
| Forecasted Market Value ( USD | $ 210.4 Million |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Australia |
| No. of Companies Mentioned | 6 |