Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Advancements in Cell and Gene Therapy Development
The rapid advancements in cell and gene therapies across the Asia-Pacific region are driving the growth of the Cell and Gene Therapy Manufacturing Quality Control (QC) market. As the region becomes a hub for cutting-edge biotech research and clinical trials, the demand for high-quality manufacturing practices to ensure the safety and efficacy of these therapies is growing.Cell and gene therapies, such as CAR-T (Chimeric Antigen Receptor T-cell) therapies and gene editing techniques like CRISPR, have shown promise in treating previously untreatable diseases, including genetic disorders and certain cancers. These therapies require stringent QC measures to verify the consistency and purity of the biologics involved, necessitating the use of advanced testing methods to assess factors like cell potency, viral vector contamination, and gene expression. As the pipeline for such therapies expands and moves closer to commercialization, the QC market is expected to see an increase in demand for testing services, equipment, and systems that ensure compliance with regulatory standards and maintain the integrity of these novel treatments.
Regulatory Push for Stringent Quality Standards
The Asia-Pacific region is experiencing heightened regulatory scrutiny as cell and gene therapies progress toward commercialization. Countries such as Japan, South Korea, and China are tightening their regulatory frameworks to ensure the safety and quality of these therapies. Regulatory agencies, including the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and China’s National Medical Products Administration (NMPA), are enforcing strict quality control requirements for the manufacturing of cell and gene therapies.As the clinical and commercial applications of these therapies grow, regulatory bodies are mandating the implementation of advanced QC protocols to monitor critical quality attributes such as sterility, genetic stability, and cell viability. This regulatory push is driving investments in state-of-the-art QC technologies and systems, including automated testing platforms, real-time monitoring, and data analytics tools to meet compliance standards. The ongoing regulatory evolution across the region is poised to accelerate the demand for high-quality manufacturing QC services and products in the Asia-Pacific market.
Key Market Challenges
Complexity and Variability in Cell and Gene Therapies
One of the major challenges facing the Asia-Pacific Cell and Gene Therapy Manufacturing QC market is the inherent complexity and variability associated with these therapies. Unlike traditional pharmaceutical products, cell and gene therapies involve living organisms, such as genetically modified cells or viral vectors, which can vary from batch to batch.The biologics involved in these therapies are subject to multiple factors, such as cell differentiation, genetic stability, and environmental conditions, which can impact the consistency and efficacy of the final product. This variability presents a challenge for quality control processes, requiring the development of specialized testing methods that can account for these dynamic and unpredictable factors. Ensuring that these therapies meet rigorous quality standards while maintaining their potency and safety over time remains a significant hurdle for manufacturers and regulators alike, making the QC process more complex and resource-intensive.
Lack of Skilled Workforce and Infrastructure
Another challenge faced by the Asia-Pacific Cell and Gene Therapy Manufacturing QC market is the shortage of skilled professionals and the lack of advanced infrastructure necessary for high-quality testing. As the demand for cell and gene therapies grows, there is an increasing need for trained personnel who can manage complex QC processes and handle the unique challenges these therapies present. The region faces a gap in qualified experts in fields like gene editing, cell biology, and biomanufacturing, which are crucial for the effective implementation of quality control measures.Additionally, there is a need for more advanced laboratories, testing equipment, and facilities capable of supporting the rigorous QC requirements of these therapies. Although countries like Japan and South Korea have made significant strides in biotechnology infrastructure, other regions in Asia-Pacific may struggle with inadequate resources to keep pace with the growing demand for high-quality cell and gene therapy products. Addressing these workforce and infrastructure challenges is critical to ensuring the continued growth and success of the QC market in this sector.
Key Market Trends
Integration of Automation and AI in QC Processes
A growing trend in the Asia-Pacific Cell and Gene Therapy Manufacturing QC market is the increasing integration of automation and artificial intelligence (AI) technologies to enhance the efficiency and accuracy of quality control processes. Traditional manual QC methods in cell and gene therapy manufacturing are often time-consuming, prone to human error, and not well-suited to the complex nature of these therapies. To address this, companies are turning to automated systems and AI-driven platforms to streamline QC procedures, reduce testing times, and improve the consistency and reliability of results.AI algorithms can analyze large datasets generated by quality testing processes, identifying patterns and trends that may not be immediately apparent to human analysts, leading to more informed decision-making. Additionally, automation can help reduce the risks of contamination and variability in the production process, ensuring that therapies meet stringent quality standards. The growing adoption of these technologies is expected to transform the QC landscape in the Asia-Pacific cell and gene therapy sector, making the process more efficient, scalable, and reliable.
Expansion of Contract Manufacturing Organizations (CMOs)
Another key trend in the Asia-Pacific market is the increasing reliance on Contract Manufacturing Organizations (CMOs) to produce cell and gene therapies, including the QC processes. As more biotechnology and pharmaceutical companies look to accelerate the development and commercialization of their therapies, many are outsourcing their manufacturing and quality control activities to CMOs with specialized expertise and infrastructure.CMOs are increasingly offering integrated services that include manufacturing, testing, and regulatory compliance, allowing companies to focus on research and development while ensuring that their therapies meet global quality standards. This trend is particularly pronounced in countries like China, India, and South Korea, where the presence of skilled labor, favorable regulatory environments, and competitive pricing make CMOs an attractive option. The rise of CMOs is expected to further fuel the demand for quality control services, as they play a critical role in ensuring that cell and gene therapies are produced and tested to meet the highest standards of safety, potency, and effectiveness.
Segmental Insights
Offering Insights
Based on the Offering, the services category is dominating the market, driven by the increasing demand for specialized quality control services essential for the development and commercialization of cell and gene therapies. As these therapies become more complex and personalized, the need for expert testing services, including viral vector testing, sterility testing, genetic stability testing, and potency assays, has risen significantly. Contract research organizations (CROs) and contract manufacturing organizations (CMOs) are offering comprehensive QC services to meet the stringent regulatory requirements for these therapies.These services help ensure that the products meet the required safety, efficacy, and quality standards, critical for gaining regulatory approval and ensuring patient safety. With the growing complexity of gene editing techniques like CRISPR and personalized cell therapies, the demand for high-level expertise in testing and quality assurance is becoming more pronounced, making the service category a key driver of growth in the Asia-Pacific market. Additionally, the integration of advanced technologies such as automation, AI, and real-time data monitoring into QC services is further contributing to the dominance of the service segment in this rapidly evolving market.
Regional Insights
China is currently dominating the Asia-Pacific Cell and Gene Therapy Manufacturing QC market, driven by its robust biotechnology sector, expanding healthcare infrastructure, and supportive government initiatives. The country has become a global leader in cell and gene therapy research and development, particularly in areas such as gene editing, CAR-T cell therapy, and regenerative medicine. China’s large population and increasing demand for advanced medical treatments have created a significant market for these innovative therapies, driving the need for high-quality manufacturing and stringent quality control processes.With the government’s strong focus on advancing biopharmaceuticals and modernizing healthcare services, China is investing heavily in state-of-the-art biotechnology facilities, including those dedicated to the production and quality testing of cell and gene therapies. Furthermore, the growing number of biotech companies and contract manufacturing organizations (CMOs) in China is accelerating the adoption of quality control services to ensure compliance with regulatory standards. The country’s relatively lower production costs, highly skilled workforce, and a favorable regulatory environment also contribute to its dominant position in the Asia-Pacific market. As China continues to expand its leadership in cell and gene therapy innovations, it is expected to remain at the forefront of the region’s manufacturing QC landscape, supporting both domestic and global therapeutic developments.
Key Market Players
- Bio-Techne Corporation
- Danaher Corporation
- F. Hoffmann-La Roche Ltd
- Lonza
- Miltenyi Biotec B.V. & Co. KG
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec
- Fujifilm Holdings Corporation
- Merck KGaA
Report Scope:
In this report, the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, By Offering:
- Products
- Services
Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, By Application:
- Safety Testing
- Potency Testing
- Identity Testing
- Stability and Genetic Fidelity Testing
- Others
Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, By Technology:
- Polymerase Chain Reaction
- Flow Cytometry
- Limulus Amebocyte Lysate (LAL)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Mass Spectrometry
- Western Blotting
- Next-Generation Sequencing
- Electrophoresis
- Others
Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, By Country:
- China
- Japan
- South Korea
- India
- Malaysia
- Indonesia
- Vietnam
- Australia
- Thailand
- Philippines
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Bio-Techne Corporation
- Danaher Corporation
- F. Hoffmann-La Roche Ltd
- Lonza
- Miltenyi Biotec B.V. & Co. KG
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec
- Fujifilm Holdings Corporation
- Merck KGaA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | January 2025 |
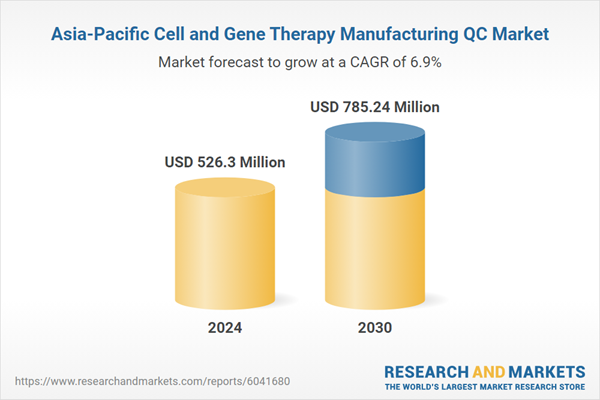
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 526.3 Million |
| Forecasted Market Value ( USD | $ 785.24 Million |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 10 |