Global Artificial Intelligence in Clinical Trials Market - Key Trends & Drivers Summarized
How Is AI Transforming Clinical Trials?
Artificial Intelligence (AI) is revolutionizing the clinical trials landscape by enhancing efficiency, reducing costs, and improving outcomes. Traditional clinical trials are often time-consuming, expensive, and fraught with inefficiencies, such as delays in patient recruitment and data collection. AI addresses these challenges by leveraging machine learning algorithms, natural language processing (NLP), and predictive analytics to streamline trial processes.In patient recruitment, AI systems analyze electronic health records (EHRs), genetic data, and other datasets to identify eligible candidates who meet trial criteria. This accelerates recruitment timelines and ensures a more diverse and representative participant pool. AI also optimizes trial design by analyzing historical data to predict the most effective protocols, endpoints, and methodologies.
During trials, AI-powered platforms enhance data collection and analysis by automating the monitoring of patient responses and identifying anomalies in real time. These tools enable faster decision-making, allowing sponsors to adjust protocols and improve trial efficiency. By addressing critical bottlenecks in trial workflows, AI is transforming clinical research and development.
What Drives the Adoption of AI in Clinical Trials?
The increasing complexity of clinical trials is a significant driver of AI adoption in this domain. With the rise of precision medicine and personalized therapies, trials require more granular data and tailored approaches to recruitment and monitoring. AI systems provide the computational power and analytical capabilities needed to manage and interpret these complex datasets, ensuring trial success.The growing cost of drug development is another key factor. On average, clinical trials account for a substantial portion of R&D expenditures in the pharmaceutical industry. AI-powered tools reduce costs by automating labor-intensive tasks, such as site selection, data analysis, and regulatory reporting. This efficiency allows sponsors to allocate resources more effectively and bring drugs to market faster.
Additionally, the demand for real-world evidence is driving the adoption of AI in post-market trials and observational studies. AI tools analyze real-world data, such as patient registries and claims databases, to assess the long-term safety and effectiveness of therapies. These capabilities align with regulatory trends emphasizing evidence-based decision-making, further supporting AI’ s integration into clinical trials.
Can AI Improve Diversity and Accessibility in Clinical Research?
AI is addressing longstanding challenges in clinical research, such as diversity and accessibility, by leveraging advanced data analysis and predictive modeling. Traditional clinical trials often struggle to recruit participants from underrepresented populations, leading to biased results that may not generalize across demographics. AI systems analyze socio-demographic data alongside clinical information to identify and recruit diverse participants, ensuring more inclusive trials.AI-powered tools are also expanding access to clinical trials by enabling decentralized and virtual trial models. These approaches reduce the need for participants to travel to trial sites, making research more accessible to those in rural or underserved areas. AI facilitates remote monitoring and data collection, allowing participants to engage from the comfort of their homes. This capability not only improves recruitment rates but also enhances the overall patient experience.
Moreover, AI enables real-time analysis of trial data, allowing sponsors to identify and address barriers to participation. By tailoring recruitment strategies and removing logistical challenges, AI is fostering a more equitable clinical research landscape. These advancements are paving the way for more representative and impactful clinical trials.
What’ s Driving the Growth of the AI in Clinical Trials Market?
The growth in the Artificial Intelligence in Clinical Trials market is driven by several key factors, reflecting the increasing demand for innovation and efficiency in clinical research. The rising prevalence of chronic diseases and the growing need for personalized therapies are spurring the adoption of AI to design and execute complex trials. AI-powered platforms accelerate recruitment, optimize protocols, and enhance data analysis, addressing critical challenges in modern clinical research.Consumer behavior trends, such as the demand for faster drug approvals and greater transparency in trial processes, are pushing pharmaceutical companies to adopt AI solutions. AI tools enable real-time reporting and monitoring, ensuring that stakeholders remain informed throughout the trial lifecycle.
Furthermore, regulatory support for the use of advanced technologies in clinical research is boosting market growth. Agencies such as the FDA are encouraging the adoption of AI to improve trial efficiency and compliance. These factors, combined with advancements in AI algorithms, data integration, and wearable technologies, are driving the rapid expansion of the AI in Clinical Trials market, positioning it as a cornerstone of future innovation in drug development and clinical research.
Report Scope
The report analyzes the Artificial Intelligence in Clinical Trials market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Component (Software Component, Services Component); Application (Drug Development Application, Drug Discovery Application, Clinical Trial Management Application, Other Applications); End-Use (Pharma & Biotech Companies End-Use, Academic and Research Institutes End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Software Component segment, which is expected to reach US$1.9 Billion by 2030 with a CAGR of a 13.3%. The Services Component segment is also set to grow at 14.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $420.6 Million in 2024, and China, forecasted to grow at an impressive 13.2% CAGR to reach $545.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Artificial Intelligence in Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Artificial Intelligence in Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Artificial Intelligence in Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABC Intelligence Inc. - VETGENUS, Antech Diagnostics, Inc., Idexx Laboratories, Inc., ImpriMed, Merck Animal Health and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Artificial Intelligence in Clinical Trials market report include:
- AiCure
- IBM Corporation
- Insilico Medicine
- IQVIA Holdings, Inc.
- Lantern Pharma Inc.
- Medidata Solutions
- Owkin, Inc.
- Phesi, Inc.
- Recursion Pharmaceuticals
- Saama Technologies, LLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AiCure
- IBM Corporation
- Insilico Medicine
- IQVIA Holdings, Inc.
- Lantern Pharma Inc.
- Medidata Solutions
- Owkin, Inc.
- Phesi, Inc.
- Recursion Pharmaceuticals
- Saama Technologies, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
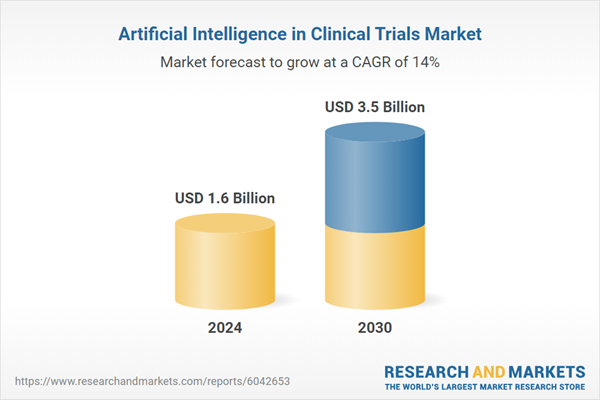
| Estimated Market Value ( USD | $ 1.6 Billion |
| Forecasted Market Value ( USD | $ 3.5 Billion |
| Compound Annual Growth Rate | 14.0% |
| Regions Covered | Global |