Peripheral vascular interventions (PVI) are minimally invasive treatments that utilize flexible devices to access blood vessels outside the heart. These procedures can open blocked vessels, remove blood clots, and manage high blood pressure. PVI represents a cutting-edge approach that minimizes the need for large incisions, resulting in faster recovery times, reduced pain, lower complication risks, and shorter hospital stays. Interventional cardiologists leverage these advanced treatment devices to enhance patient outcomes. The peripheral vascular intervention market is expanding primarily due to the rising prevalence of peripheral artery disease (PAD) and advancements in technology. This growth is further supported by an increasing preference for minimally invasive procedures, an aging population, and higher healthcare spending. PAD restricts blood flow to the limbs and is becoming more common due to factors like diabetes, obesity, and smoking. Innovative devices such as drug-eluting stents, atherectomy devices, and embolization particles have improved performance and patient outcomes. Additionally, the growing elderly population and increased healthcare expenditure are directly contributing to the demand for these treatments.
Drivers of the Peripheral Vascular Intervention Market:
- Rising Prevalence of Peripheral Arterial Disease (PAD): The increasing incidence of PAD is a significant factor driving the growth of the peripheral vascular intervention market. PAD occurs when arteries supplying blood to the limbs become narrowed or blocked by plaque, leading to symptoms such as leg pain and poor wound healing. The prevalence of PAD is rising due to unhealthy lifestyles, which in turn increases the demand for targeted treatments. There is a notable shift toward minimally invasive therapies like angioplasty and stenting, along with advancements in interventional techniques and device technologies.
Geographical Outlook of the Peripheral Vascular Intervention Market:
- North America Anticipated to Hold Significant Market Share: The peripheral vascular intervention market is segmented into North America, South America, Europe, the Middle East & Africa, and Asia-Pacific regions. North America is expected to dominate this market due to a strong demand for renewable energy solutions and government incentives promoting sustainability. The U.S. market is particularly robust due to rising obesity rates that encourage gym memberships and healthier lifestyles.
Reasons for buying this report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape up future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decision to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive Intelligence.Report Coverage:
- Historical data & forecasts from 2022 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, Customer Behaviour, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others)
The Peripheral Vascular Intervention Market has been segmented as following:
- By Product Type
- Stents
- Balloons
- Atherectomy System
- Catheters
- Peripheral Accessories
- Guidewires
- Others
- By Application
- Peripheral Artery Disease
- Deep Vein Thrombosis (DVT)
- Aneurysms
- Venous Disease
- Pulmonary Embolism (PE)
- Others
- By End-User
- Hospitals
- Ambulatory Surgical Centers
- Others
- By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- Japan
- India
- South Korea
- Indonesia
- Thailand
- Taiwan
- Others
- North America
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- Abbott Laboratories
- BD (Becton, Dickinson, and Company)
- Zylox-Tonbridge Medical Technology Co., Ltd.
- BIOTRONIK
- Medtronic
- Terumo Corporation
- B. Braun Melsungen AG
- Cook Medical
- Meril Life Sciences Pvt. Ltd.
- Merit Medical Systems
- Philips
- TE Connectivity
- AngioDynamics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | January 2025 |
| Forecast Period | 2025 - 2030 |
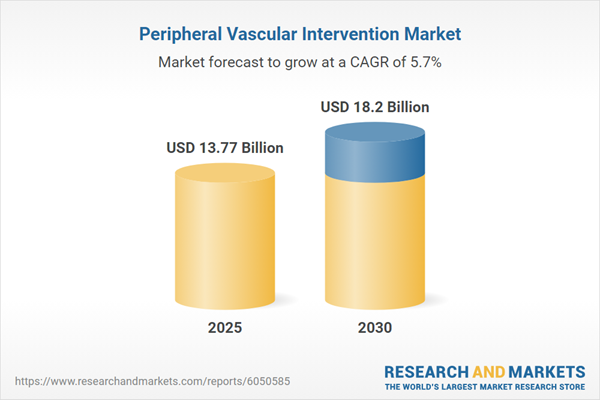
| Estimated Market Value ( USD | $ 13.77 Billion |
| Forecasted Market Value ( USD | $ 18.2 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |