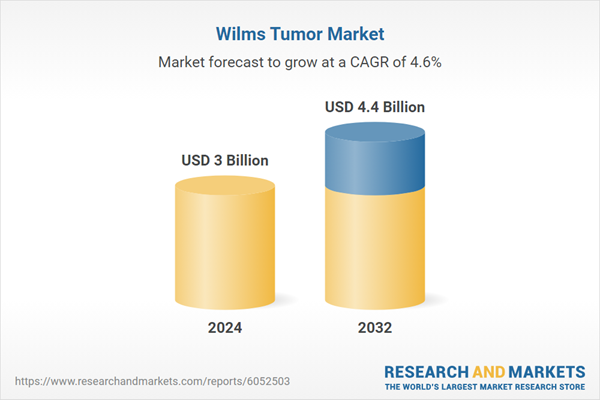
Wilms Tumor Market Analysis
Wilms tumor, also known as nephroblastoma, is a rare type of kidney cancer that primarily affects children, usually between the ages of 3 and 4. Early diagnosis and advancements in treatment, such as surgery, chemotherapy, and radiation therapy, have significantly improved survival rates. The market for Wilms tumor treatments is growing due to increasing awareness, advancements in medical technologies, and ongoing research into more effective and targeted therapies.Market Drivers
- Increasing Incidence of Paediatric Cancer: The rising incidence of Wilms tumor, particularly in paediatric populations, is driving demand for more effective treatment options. Early diagnosis and intervention have become more common due to increased awareness and medical advancements, resulting in higher demand for treatments such as surgery and chemotherapy.
- Advancements in Treatment Modalities: Innovations in targeted therapies, immunotherapy, and gene therapy are enhancing treatment outcomes for Wilms tumour patients. These advanced treatment modalities offer improved efficacy and fewer side effects compared to traditional therapies, contributing to the growth of the Wilms tumour market.
- Rising Investment in Cancer Research: Increased funding and investment in cancer research, particularly in paediatric oncology, are driving the development of novel therapies for Wilms tumor. Governments, non-profit organisations, and pharmaceutical companies are investing in research initiatives to discover new and more effective treatment options for this rare disease.
- Favourable Regulatory Support: Favourable regulatory policies and accelerated approval processes for rare paediatric cancers are promoting the faster introduction of innovative therapies. Regulatory bodies such as the FDA and EMA are providing support to pharmaceutical companies in the development of treatments for rare diseases like Wilms tumour, encouraging market growth.
- Growing Awareness of Paediatric Cancer Treatments: Increasing public and healthcare professional awareness about Wilms tumour and other paediatric cancers is driving earlier diagnosis and treatment. Campaigns and educational initiatives by healthcare organisations are leading to improved detection rates and a greater demand for treatment options.
Challenges
- Limited Access to Advanced Therapies in Developing Regions: Access to advanced medical technologies and treatments remains limited in developing countries due to inadequate healthcare infrastructure and financial constraints. This lack of access to modern therapies restricts the growth of the Wilms tumour market in certain regions.
- Adverse Effects of Traditional Therapies: While chemotherapy and radiation therapy are effective in treating Wilms tumor, they often come with severe side effects such as hair loss, nausea, and long-term complications like secondary cancers. These adverse effects may limit the use of traditional therapies, creating a demand for safer alternatives.
- Delayed Diagnosis in Low-Income Regions: In low-income regions, delayed diagnosis of Wilms tumour remains a significant challenge. A lack of awareness and limited access to diagnostic facilities can result in late-stage diagnosis, reducing the efficacy of available treatments and negatively impacting patient survival rates.
- Stringent Regulatory Approvals: Stringent regulatory approval processes for new drugs and treatments pose a challenge for pharmaceutical companies. Meeting the high standards of safety and efficacy set by regulatory authorities can delay the introduction of innovative therapies, affecting the market’s growth.
Future Opportunities
- Development of Personalised Medicine: The growing interest in personalised medicine, which tailors treatment to individual patients based on genetic and molecular factors, offers significant potential in the Wilms tumour market. Personalised treatments, such as gene therapy, provide targeted interventions that improve patient outcomes and reduce side effects.
- Partnerships and Collaborations in Research: Collaborations between pharmaceutical companies, research institutions, and non-profit organisations are driving the development of new therapies for Wilms tumor. Such partnerships allow for the sharing of resources, expertise, and technology, accelerating the pace of research and innovation in the market.
- Focus on Paediatric Oncology Centres: The development of specialised paediatric oncology centres is increasing access to advanced therapies for children with Wilms tumor. These centres focus on providing comprehensive care, including state-of-the-art diagnostic and treatment options, driving the market for specialised Wilms tumour treatments.
- Expansion of Immunotherapy Applications: Immunotherapy, which harnesses the body’s immune system to fight cancer, is showing promise in treating Wilms tumor. Continued research into immunotherapy applications for paediatric cancers is expected to provide new treatment options, creating opportunities for market growth.
Wilms Tumor Market Trends
The industry is evolving rapidly, driven by technological innovations and advancements in various practices. As continuous research uncovers new insights across sectors, several key trends are emerging, shaping the future direction of the market. These trends are expected to significantly influence the landscape, improving outcomes, enhancing precision, and expanding access to advanced solutions across products, therapies, and services.- Increasing Use of Targeted Therapy
- Growing Adoption of Minimally Invasive Surgery
- Focus on Long-Term Survivorship
- Integration of Artificial Intelligence in Oncology
- Advances in Gene Therapy for Paediatric Cancer
- Rising Demand for Non-Invasive Diagnostic Techniques
Wilms Tumor Market Segmentation
Market Breakup by Treatment
- Surgery
- Chemotherapy
- Radiation Therapy
- Targeted Therapy
- Immunotherapy
- Gene Therapy
Market Breakup by Stages
- Stage I and II
- Stage III
- Stage IV
- Stage V
Market Breakup by Diagnosis
- Imaging Tests
- Lab Tests
Market Breakup by End User
- Hospitals
- Diagnostic Laboratories
- Cancer Research Institutes
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Wilms Tumor Market Competitive Landscape
Key players in the Wilms tumor market include NexImmune Inc., Endeavor Biomedicines, Inc., Acrotech Biopharma Inc., MacroGenics, Inc., Cell Medica Ltd, Cue Biopharma, Inc., F. Hoffmann-La Roche AG, Sumitomo Pharma America, Inc., Astellas Pharma Inc., Cellectar Biosciences, Inc., and Sellas Life Sciences Group. These companies are focusing on developing innovative therapies, including immunotherapy and gene therapy, to enhance treatment options for Wilms tumor. Strategic collaborations, clinical trials, and product launches are key strategies to strengthen their market presence.Key Questions Answered in the Report
- What are the primary drivers contributing to the growth of the Wilms tumor market?
- How are advancements in targeted therapies influencing the treatment of Wilms tumor?
- What challenges do healthcare providers face in the treatment of Wilms tumor in developing regions?
- Which regions are expected to see the highest growth in the Wilms tumor market?
- How does gene therapy hold promise for treating advanced stages of Wilms tumor?
- What are the most commonly used treatment options for early-stage Wilms tumor?
- How are non-invasive diagnostic techniques shaping the diagnosis and treatment of Wilms tumor?
- What role does AI play in enhancing patient care for Wilms tumor?
- How are partnerships and collaborations driving research into new treatments for Wilms tumor?
- What impact does the high cost of treatment have on access to care for Wilms tumor patients?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the wilms tumor market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Wilms tumor market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders analyze the level of competition within the wilms tumor industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- NexImmune Inc.
- Endeavor Biomedicines, Inc.
- Acrotech Biopharma Inc
- MacroGenics, Inc.
- Cell Medica Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | January 2025 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 3 Billion |
| Forecasted Market Value ( USD | $ 4.4 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |