Von Hippel-Lindau Disease Market Analysis
Von Hippel-Lindau disease is a rare genetic disorder characterised by the formation of tumors and cysts in various parts of the body. These tumors can be benign or malignant and often affect areas such as the kidneys, pancreas, and nervous system. Early detection and treatment are essential, as Von Hippel-Lindau-related tumors can lead to severe complications, including cancer. Genetic mutations in the Von Hippel-Lindau gene are responsible for this condition, and it requires ongoing management through surveillance and various therapeutic approaches.Market Drivers
- Increasing Genetic Testing and Early Diagnosis: The growing availability of genetic testing has significantly enhanced the early diagnosis of Von Hippel-Lindau disease. This rise in early detection increases demand for treatments and therapeutic interventions, leading to market growth.
- Advancements in Precision Medicine: New advances in precision medicine allow for highly personalised treatment approaches for Von Hippel-Lindau disease, targeting specific genetic mutations, driving demand for tailored therapies and better patient outcomes.
- Rise in Tumour-Specific Treatments: The focus on developing therapies specifically targeting tumors associated with Von Hippel-Lindau disease, particularly renal cell carcinomas, and hemangioblastomas, is leading to increased treatment options and market expansion.
- Growing Awareness of Rare Genetic Disorders: Enhanced awareness of rare genetic conditions like Von Hippel-Lindau among the public and healthcare professionals is boosting early diagnosis and proactive management, further driving the demand for advanced treatments.
- Expanding Access to Specialised Healthcare: Improved access to healthcare infrastructure in developing markets is making advanced treatments more accessible to patients, thereby driving market growth.
Challenges
- High Cost of Therapies: Treatments for Von Hippel-Lindau, especially precision medicines, are expensive, making them less accessible in lower-income regions and creating a financial burden on healthcare systems and patients.
- Complex Disease Management: The multifaceted nature of Von Hippel-Lindau disease, with its diverse tumor manifestations, makes treatment complicated and resource-intensive, requiring specialised care across multiple disciplines.
- Low Awareness in Developing Markets: In regions with limited resources, awareness of rare genetic disorders like Von Hippel-Lindau remains low, which delays diagnosis and treatment, resulting in poorer patient outcomes.
- Therapeutic Side Effects: Some targeted therapies and precision medicines used to manage Von Hippel-Lindau can cause side effects, leading to patient non-compliance or discontinuation of treatment.
Future Opportunities
- Rising Investment in Personalised Medicine: Investment in personalised medicine is growing, providing opportunities for the development of more effective, patient-specific treatments for Von Hippel-Lindau disease, which could lead to better outcomes.
- Collaboration in Research and Development: Increased collaboration between pharmaceutical companies, research institutions, and government bodies provides significant opportunities for the development of innovative treatments, including targeted therapies.
- Emerging Diagnostic Tools: Innovations in diagnostic tools, such as advanced imaging techniques and biomarker-based screenings, present opportunities for earlier detection and more accurate diagnoses, improving overall patient care.
- Growth in Telemedicine Platforms: The expansion of telemedicine allows Von Hippel-Lindau patients in remote or underserved areas to access specialised healthcare services and consultations, thereby improving the management of this complex genetic disorder.
Von Hippel-Lindau Disease Market Trends
The industry is evolving rapidly, driven by technological innovations and advancements in various practices. As continuous research uncovers new insights across sectors, several key trends are emerging, shaping the future direction of the market. These trends are expected to significantly influence the landscape, improving outcomes, enhancing precision, and expanding access to advanced solutions across products, therapies, and services.- Development of Targeted Therapies
- Increased Focus on Early Diagnosis
- Advances in Surgical Techniques
- Precision Oncology
- Collaboration in R&D
- Growing Use of Artificial Intelligence in Diagnostics
Von Hippel-Lindau Disease Market Segmentation
Market Breakup by Drug Class
- Tyrosine Kinase Inhibitors (TKIs)
- VEGF Inhibitors
- HIF-2α Inhibitors
- Immunotherapy
- Others
Market Breakup by Tumor Site
- Brain
- Spinal Cord
- Eyes
- Kidneys
- Adrenal Glands
- Pancreas
- Liver
- Lungs
- Reproductive Tract
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
- Others
Market Breakup by End User
- Hospitals and Specialty Clinics
- Cancer Treatment Centers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Von Hippel-Lindau Disease Market Competitive Landscape
Companies like Merck & Co., Inc., Bristol Myers Squibb Company, Novartis Pharmaceuticals, and Exelixis, Inc. are key players in the VHL disease market, actively investing in R&D to develop novel targeted therapies, including VEGF inhibitors and immunotherapies. These companies are leading efforts in clinical trials to improve treatment outcomes for patients with Von Hippel-Lindau-related tumors. Expansion in gene therapy research is also positioning these firms as innovators in the Von Hippel-Lindau space.Key Questions Answered in the Report
- What is the expected CAGR of the Von Hippel-Lindau disease market during 2024-2032?
- Which drug class dominates the Von Hippel-Lindau disease treatment market?
- How does genetic screening impact the diagnosis of Von Hippel-Lindau?
- What are the key challenges facing the Von Hippel-Lindau treatment market?
- Which regions lead the global Von Hippel-Lindau disease market?
- How is personalised medicine influencing treatment trends in the Von Hippel-Lindau market?
- What role do VEGF inhibitors play in treating Von Hippel-Lindau-related tumors?
- What advancements in gene therapy are emerging for Von Hippel-Lindau patients?
- How does telemedicine contribute to the management of Von Hippel-Lindau disease?
- What are the primary treatment options for Von Hippel-Lindau-associated renal cell carcinoma?
- How are pharmaceutical companies collaborating to advance Von Hippel-Lindau therapies?
- Which disease manifestation of Von Hippel-Lindau is most prevalent in patients?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Von Hippel-Lindau disease market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Von Hippel-Lindau disease market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders analyze the level of competition within the Von Hippel-Lindau disease industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Merck & Co., Inc.
- Bristol Myers Squibb Company
- Novartis Pharmaceuticals
- Exelixis, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | January 2025 |
| Forecast Period | 2024 - 2032 |
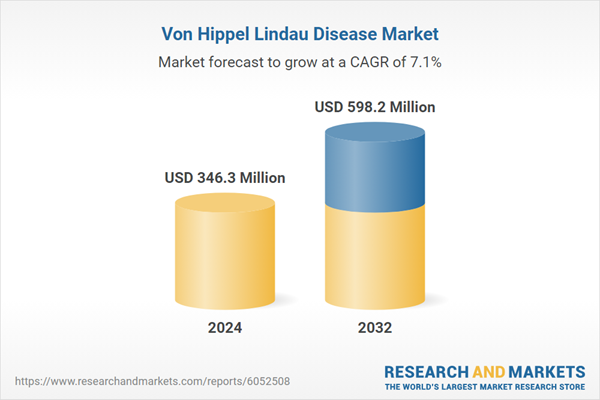
| Estimated Market Value ( USD | $ 346.3 Million |
| Forecasted Market Value ( USD | $ 598.2 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |