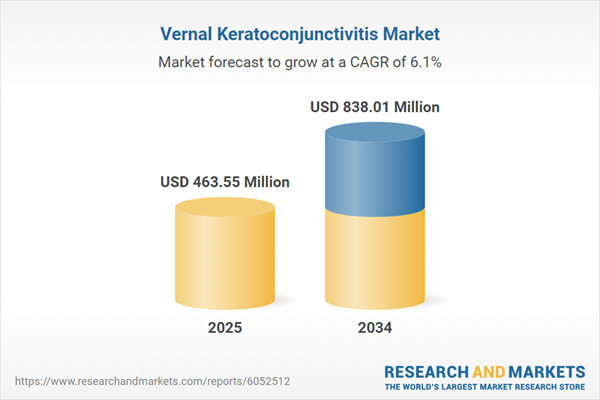
Vernal Keratoconjunctivitis Market Overview
Vernal keratoconjunctivitis is a long-lasting allergic condition triggered by sensitivity to allergens found in the air. Younger males are the ones that are more commonly affected, showing symptoms like itching, sensitivity to light, redness, and discharge of mucus. The rise in vernal keratoconjunctivitis cases, especially in adults, is driving the market growth. Additionally, there is a growing preference for using immunomodulatory therapies, which are poised to impact the market dynamics in the forecast period.Vernal Keratoconjunctivitis Market Growth Drivers
Increasing Recognition of Vernal Keratoconjunctivitis Cases in Adults to Drive Market Growth
The market for vernal keratoconjunctivitis is expanding as more adult cases are identified. A United States-based study demonstrated that approximately 10% of cases persist into adulthood or arise post-puberty, broadening the patient population and necessitating new treatments to address issues, thereby stimulating market expansion.Vernal Keratoconjunctivitis Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increasing Acquisition Events
The acquisition trend, such as Harrow acquiring Santen’s ophthalmic portfolio that includes VERKAZIA®, shows market consolidation. Major companies are enhancing their portfolios to offer all-inclusive solutions for eye care, which is set to positively influence market growth.Shift Toward Immunomodulatory Therapies
Immunomodulatory therapies, like Cyclosporine A, are trending in the market for treating vernal keratoconjunctivitis. This shift focuses on addressing immune mechanisms rather than just symptoms, in line with a broader move towards safer, more targeted therapies.Advances in Treatment Options
The introduction of specialized treatments such as Verkazia® by Santen Pharmaceutical Co., Ltd, a Japanese pharmaceutical company, for patients suffering from vernal keratoconjunctivitis is a major driver of growth, meeting medical needs and increasing opportunities for innovation in the market.Growth in Allergy and Immunology Clinics
A significant market trend is the expansion of specialty clinics in allergology and immunology, which is improving access to medical care for vernal keratoconjunctivitis patients, especially in regions with higher prevalence. Such clinics offer targeted treatments that have the potential to reduce the progression of symptoms more effectively and are anticipated to accommodate the growing market demand for advanced vernal keratoconjunctivitis therapies.Vernal Keratoconjunctivitis Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Treatment:
- Nonsteroidal Anti-inflammatory Drugs - NSAIDs
- Mast Cell Stabilizers
- Antihistamines
- Topical Corticosteroids
- Cyclosporine
- Others
Market Breakup by Dosage Form:
- Ointment
- Gel
- Tablets
- Others
Market Breakup by Distribution Channel:
- Hospitals and Clinics
- Online Pharmacies
- Retail Pharmacies
- Others
Market Breakup by Region:
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Vernal Keratoconjunctivitis Market Share
Segmentation Based on Treatment to Witness Substantial Growth
Based on the treatment, the market is segmented into nonsteroidal anti-inflammatory drugs - NSAIDs, mast cell stabilizers, antihistamines, topical corticosteroids, cyclosporine, and others. Topical corticosteroids are expected to dominate the market share as they remain the most widely used option due to their effectiveness in rapidly reducing inflammation and alleviating symptoms like itching, pain, and sensitivity to light.Vernal Keratoconjunctivitis Market Analysis by Region
Based on region, the market report covers the United States, EU-4 (Germany, France, Italy, Spain), United Kingdom, Japan, and India. The United States is one of the leading markets owing to the high prevalence of conjunctivitis, coupled with an increased demand for effective treatments. With improved healthcare services and accessibility, the United States market for vernal keratoconjunctivitis is projected to witness significant growth in the coming years.Leading Players in the Vernal Keratoconjunctivitis Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Santen Pharmaceutical Co., Ltd
Santen Pharmaceutical, is a Japanese company specialized specializing in ophthalmology, was granted FDA authorization in June 2021, for Verkazia, an eye drop medication containing cyclosporine, to manage vernal keratoconjunctivitis.Novartis Pharmaceuticals Corporation
Novartis is a cutting-edge pharmaceutical company that introduced ALOMIDE eye drops for managing vernal keratoconjunctivitis, stopping allergic responses by inhibiting mast cells from releasing inflammatory substances.Senju Pharmaceutical Co., Ltd
Senju Pharmaceutical Co., Ltd, headquartered in Japan, has a prominent presence in the market. The company specializes in ophthalmology, boasting a robust portfolio of medications for managing allergic conjunctivitis and vernal keratoconjunctivitis.Other key players in the market include Akari Therapeutics Plc., Allakos Inc., Satellos Bioscience Inc., Viatris Inc., Aldeyra Therapeutics, Inc., Laboratoires Thea S.A.S., and Astellas Pharma Inc
Key Questions Answered in the Vernal Keratoconjunctivitis Market
- What was the vernal keratoconjunctivitis market value in 2024?
- What is the vernal keratoconjunctivitis market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on the treatment?
- What is the market breakup based on the dosage form?
- What is the market breakup based on the distribution channel?
- What are the major factors aiding the vernal keratoconjunctivitis market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the vernal keratoconjunctivitis market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Santen Pharmaceutical Co., Ltd.
- Novartis Pharmaceuticals Corporation
- Senju Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 463.55 Million |
| Forecasted Market Value ( USD | $ 838.01 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 3 |