Speak directly to the analyst to clarify any post sales queries you may have.
The Progesterone ELISA Test Kits Market is undergoing sustained growth, shaped by evolving diagnostic requirements and technological advances. As clinical laboratories, fertility clinics, research institutions, and life sciences companies prioritize precision and efficiency, demand for these assay kits continues to accelerate, fueling market expansion across diverse healthcare landscapes.
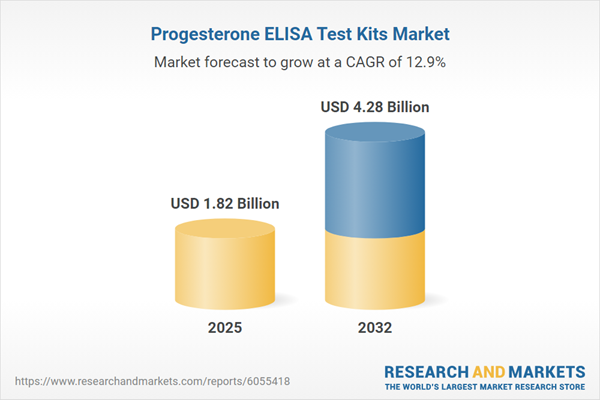
Market Snapshot: Progesterone ELISA Test Kits
This market is advancing on a strong upward trajectory, reflecting strategic investments by healthcare providers and research organizations. The sector benefits from increasing fertility assessments, expanded endocrine disorder diagnostics, and robust R&D activities. Steady advances in ELISA technologies and rising focus on healthcare infrastructure, particularly in emerging regions, are powering the market’s healthy compound annual growth rate. These dynamics point to stable long-term prospects for market participants, with attractive opportunities for innovation and market capture.
Scope & Segmentation
- Kit Types: Includes coated and uncoated ELISA kits, meeting diverse workflow and customization needs.
- Test Types: Coverage of Direct, Indirect, and Sandwich ELISA formats, suitable for both sensitive diagnostics and broader screening applications.
- Sample Types: Kits validated for serum, plasma, saliva, urine, and whole blood, supporting both routine and specialized use cases.
- Technology Platforms: Integration of chemiluminescent, colorimetric, and fluorescent detection methods to balance sensitivity, throughput, and affordability.
- End Users: Encompasses biotechnology companies (including biopharma and CROs), clinical laboratories (hospital-based and private), fertility clinics (IVF and obstetrics), pharmaceutical companies (generic and innovative), and research institutions (government and universities).
- Distribution Channels: Broad access via offline (direct sales, distributors) and online channels (company websites, third-party portals).
- Regional Coverage: In-depth analysis for the Americas (with North and Latin American detail), Europe, Middle East & Africa (including Western Europe, Gulf states, Africa), and Asia-Pacific (featuring prominent and growth-focused countries).
- Profiled Companies: In-depth evaluation includes Abbexa Ltd, Abcam PLC, Arbor Assays, Assay Genie, Aviva Systems Biology Corporation, Biorbyt Ltd, Cayman Chemical Company, Cepham Life Sciences, Crystal Chem Inc., CUSABIO Technology, DRG International, Eagle Biosciences, ELK Biotechnology, Enzo Biochem, FineTest Biotech, LifeSpan BioSciences, Neogen Corporation, Novus Biologicals, RayBiotech, Salimetrics, Signalway Antibody, Thermo Fisher Scientific, Universal Biotechnolgy, VWR International, and XpressBio, among others.
Key Takeaways for Decision-Makers
- Progesterone ELISA test kits play a pivotal role in fertility monitoring, therapeutic drug development, and routine clinical diagnostics, supporting workflows across laboratory and research environments.
- Innovations in chemiluminescent and fluorescent detection have significantly elevated assay sensitivity and multiplexing capacity, while miniaturized colorimetric systems have expanded point-of-care capabilities.
- Technology adoption is closely linked to end-user needs, with coated kits valued for standardized protocols and uncoated formats offering flexibility in research applications.
- Shifting market access strategies feature growth in digital procurement, vertical integration between manufacturers and end users, and expansion through direct and third-party online channels.
- Competition is influenced by advances in assay reagents, strategic alliances, and geographic expansion via local partnerships and digital service platforms.
- Regional opportunities are shaped by regulatory environments, donor-funded initiatives, and investment in healthcare modernization projects, especially within emerging markets.
Tariff Impact: Navigating 2025 U.S. Trade Challenges
The revised United States tariff regime has introduced new cost and compliance complexities for manufacturers and distributors. In response, many companies are exploring localized raw material sourcing, investing in regional manufacturing, and optimizing inventory strategies to manage supply chain risks. Pricing models are evolving to absorb duty fluctuations and maintain end-user affordability, underscoring the importance of flexible and transparent procurement approaches.
Methodology & Data Sources
This report draws on a combination of primary and secondary research, including direct interviews with laboratories, procurement leaders, and fertility experts. Comprehensive reviews of peer-reviewed literature and regulatory documents enhance data reliability. All findings are validated through triangulation and independent analyst reviews, ensuring that conclusions align with current industry realities.
Why This Report Matters
- Offers actionable insights tailored to diagnostic leaders, procurement teams, and growth-focused executives navigating a dynamic healthcare sector.
- Delivers deep segmentation analysis and competitive context to inform strategic planning, Product selection, and partnership development.
- Empowers organizations to adjust supply chain, investment, and market entry strategies in response to regulatory, technical, and regional developments.
Conclusion
Stakeholders in the progesterone ELISA test kits market must remain agile as innovation, regulations, and procurement models evolve. Strategic investment in technologies, regional partnerships, and customer-centric solutions will define long-term growth and success within this dynamic global sector.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Progesterone ELISA Test Kits market report include:- Abbexa Ltd
- Abcam PLC
- Arbor Assays, Inc.
- Assay Genie
- Aviva Systems Biology Corporation
- Biorbyt Ltd
- Cayman Chemical Company
- Cepham Life Sciences, Inc.
- Crystal Chem Inc.
- CUSABIO Technology LLC
- DRG International, Inc.
- Eagle Biosciences, Inc.
- ELK Biotechnology CO.,Ltd.
- Enzo Biochem, Inc.
- FineTest Biotech Inc.
- LifeSpan BioSciences, Inc.
- Neogen Corporation
- Novus Biologicals, LLC
- RayBiotech, Inc.
- Salimetrics, LLC.
- Signalway Antibody LLC
- Thermo Fisher Scientific Inc.
- Universal Biotechnolgy Private Limited
- VWR International, LLC.
- XpressBio, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.82 Billion |
| Forecasted Market Value ( USD | $ 4.28 Billion |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |