The growing number of traumatic injuries from road accidents, falls, and violence is a key factor fueling the U.S. REBOA market. This surge in critical cases increases the demand for effective emergency interventions such as REBOA. As a result, advancements and adoption of these life-saving devices continue to expand. According to the U.S. Bureau of Labor Statistics (BLS), in 2022, there were 5,486 fatal work injuries in the United States, marking a 5.7% increase from 2021. Transportation incidents were the leading cause, accounting for 37.7% of these fatalities. In 2023, the number of fatal work injuries decreased by 3.7% to 5,283, with transportation incidents remaining the most frequent cause, comprising 36.8% of fatalities.
Advancements in medical technology have greatly improved the safety and effectiveness of REBOA procedures. Innovations such as enhanced balloon designs, advanced catheter systems, and improved imaging allow for more precise placement and monitoring. These improvements lead to better patient outcomes and increased adoption of REBOA in trauma care. For instance, in June 2024, Utah-based Emergency Scientific announced the first use of its Landmark REBOA catheter after receiving FDA 510(k) clearance. The device is designed for temporary occlusion of large vessels to control emergency hemorrhages. Its advanced design improves occlusion precision while reducing complications compared to traditional methods.
Increasing healthcare spending is fueling the expansion of the U.S. REBOA industry. Higher investments in advanced medical technologies and trauma care are boosting the adoption of REBOA devices. This growth supports better emergency interventions and improved patient outcomes. According to the Centers for Medicare & Medicaid Services, in 2023, healthcare spending in the U.S. reached USD 4.9 trillion, marking a 7.5% increase, up from 4.6% in 2022. Medicare and private health insurance spending grew faster than in 2022. In 2023, the health sector accounted for 17.6% of the economy, comparable to 17.4% in 2022, but this figure is lower than the shares recorded in 2020 and 2021 at the height of the COVID-19 pandemic.
U.S. Resuscitative Endovascular Balloon Occlusion Of The Aorta Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, the analyst has segmented the U.S. resuscitative endovascular balloon occlusion of the aorta market report based on end use:End Use Outlook (Revenue, USD Million, 2018-2030)
- Aortic Occlusion
- Cardiac Arrest
- Tactical Combat Casualty Care
This report addresses:
- Market intelligence to enable effective decision-making.
- Market estimates and forecasts from 2018 to 2030.
- Growth opportunities and trend analyses.
- Segment and regional revenue forecasts for market assessment.
- Competition strategy and market share analysis.
- Product innovation listings for you to stay ahead of the curve.
- COVID-19's impact and how to sustain in this fast-evolving market.
Why Should You Buy This Report?
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The major companies profiled in this U.S. Resuscitative Endovascular Balloon Occlusion of The Aorta market report include:- Prytime Medical Devices, Inc.
- Emergency Scientific, LLC
- Qx Médical
- Front Line Medical Technologies
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 80 |
| Published | February 2025 |
| Forecast Period | 2024 - 2030 |
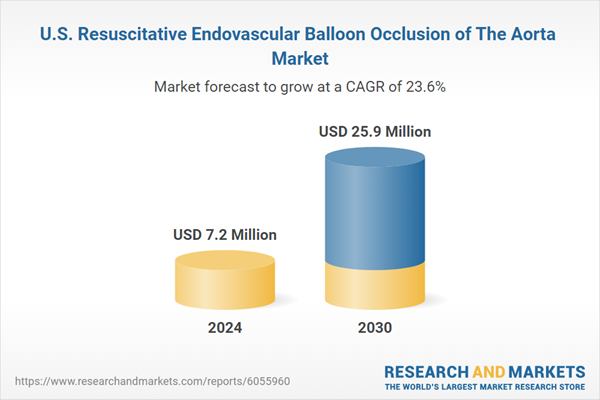
| Estimated Market Value ( USD | $ 7.2 Million |
| Forecasted Market Value ( USD | $ 25.9 Million |
| Compound Annual Growth Rate | 23.6% |
| Regions Covered | United States |
| No. of Companies Mentioned | 4 |