Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
One of the primary factors contributing to the growth of the outsourcing market is the rising complexity of pharmaceutical and biotech development. Companies are increasingly relying on Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) to provide the necessary technical expertise and infrastructure required to support these advanced projects. By outsourcing these functions, companies can streamline operations, reduce overhead costs, and focus on their core competencies such as innovation and product development.
Key Market Drivers
Growth in Healthcare Industry
The rapid expansion of Saudi Arabia’s healthcare industry has emerged as a key driver of the country’s biotechnology and pharmaceutical services outsourcing market. As part of its Vision 2030 initiative, Saudi Arabia is making substantial investments to enhance its healthcare infrastructure, modernize medical services, and improve the overall quality of healthcare delivery. This growth has created new opportunities for biotechnology and pharmaceutical companies to outsource specialized services, ranging from research and development (R&D) to clinical trials and regulatory compliance.One of the main factors contributing to this trend is the increasing demand for innovative treatments and biopharmaceutical products in Saudi Arabia. Saudi Arabia's ambitious vision for advancing medical technologies encompasses the integration of artificial intelligence (AI) systems in diagnostics, the development of telemedicine platforms for remote patient care, and the promotion of precision medicine for personalized treatments. Additionally, the nation is heavily investing in robotics and automation technologies for surgical procedures and patient care, as well as in digital health record systems to enhance data management and streamline healthcare services.
Key Market Challenges
Limited Local Contract Research Organizations (CROs)
One of the key challenges facing the Saudi Arabia biotechnology and pharmaceutical services outsourcing market is the limited presence of local Contract Research Organizations (CROs). As demand for outsourcing services continues to rise, the scarcity of established, local CROs has created a significant gap in the market, posing obstacles for companies seeking specialized research and development (R&D) support within the Kingdom.The shortage of local CROs forces many biotechnology and pharmaceutical companies to rely on international service providers, which can lead to higher operational costs and longer project timelines due to logistical complexities. Additionally, international partnerships may lack the familiarity with local regulations, market dynamics, and cultural nuances that are essential for smooth project execution and regulatory compliance in Saudi Arabia.
Key Market Trends
Expansion of Clinical Trials and Regulatory Services
The expansion of clinical trials and regulatory services has become a notable trend driving growth in Saudi Arabia’s biotechnology and pharmaceutical services outsourcing market. As the country continues to invest in the development of its healthcare and pharmaceutical sectors, there is a rising need for specialized outsourcing partners that can support clinical trials, ensure compliance with regulatory requirements, and expedite the approval of innovative treatments.The Clinical Trials Administration is committed to delivering its services with professionalism, focusing on the evaluation, registration, and monitoring of clinical trials conducted in Saudi Arabia. Its efforts contribute to safeguarding the well-being of clinical trial participants while enhancing the expertise of investigators in the field of clinical research. Additionally, the administration aims to strengthen the regulatory body's capacity, as well as its legislative and oversight functions. The administration also oversees inspections of clinical trial sites, ensuring that researchers and research organizations adhere to all aspects of the study protocol and meet the requirements outlined in Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) guidelines.
Key Market Players
- Intertek Group Plc
- SGS Société Générale de Surveillance SA.
- Charles River Laboratories International, Inc.
- Eurofins Saudi Ajal Laboratories
- MERCK LIMITED
Report Scope
In this report, the Saudi Arabia Biotechnology And Pharmaceutical Services Outsourcing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Saudi Arabia Biotechnology And Pharmaceutical Services Outsourcing Market, By Service:
- Consulting
- Regulatory Affairs
- Product Design & Development
- Auditing and Assessment
- Product Maintenance
- Training & Education
- Others
Saudi Arabia Biotechnology And Pharmaceutical Services Outsourcing Market, By End User:
- Pharmaceutical Companies
- Biotech Companies
Saudi Arabia Biotechnology And Pharmaceutical Services Outsourcing Market, By Region:
- Eastern
- Western
- Northern & Central
- Southern
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Saudi Arabia Biotechnology And Pharmaceutical Services Outsourcing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Intertek Group Plc
- SGS Société Générale de Surveillance SA.
- Charles River Laboratories International, Inc.
- Eurofins Saudi Ajal Laboratories
- MERCK LIMITED
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
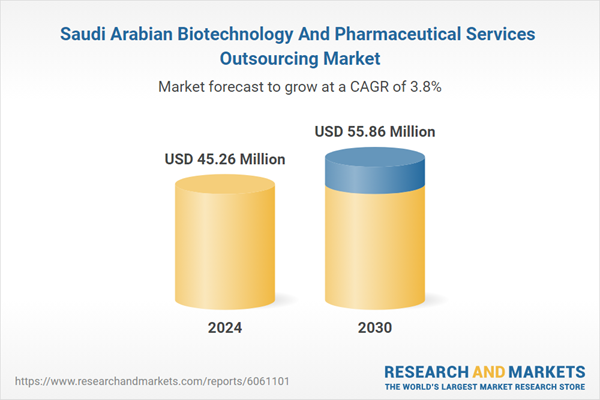
| Estimated Market Value ( USD | $ 45.26 Million |
| Forecasted Market Value ( USD | $ 55.86 Million |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Saudi Arabia |
| No. of Companies Mentioned | 5 |