Global Gastroretentive Drug Delivery Systems Outsourcing Market - Key Trends & Drivers Summarized
The outsourcing of gastroretentive drug delivery systems (GRDDS) is gaining significant traction as pharmaceutical companies seek to improve bioavailability and therapeutic efficacy of drugs with narrow absorption windows or limited solubility in the upper gastrointestinal (GI) tract. GRDDS technologies such as floating systems, bioadhesive systems, swelling and expanding systems, and high-density systems are highly specialized and require extensive formulation expertise and advanced infrastructure. As a result, many drug developers are partnering with contract development and manufacturing organizations (CDMOs) that possess the technical capabilities and regulatory know-how to bring these complex formulations to market efficiently.Key trends influencing the GRDDS outsourcing market include rising demand for extended-release oral formulations, increasing focus on patient-centric drug delivery, and the need to differentiate products in a competitive therapeutic landscape. The adoption of GRDDS is particularly valuable for drugs treating chronic gastrointestinal disorders, cardiovascular diseases, and type 2 diabetes, where prolonged drug residence time enhances therapeutic outcomes. As major pharmaceutical firms streamline internal operations to focus on core R&D, outsourcing non-core competencies such as GRDDS formulation, scale-up, and manufacturing has become a strategic imperative. Furthermore, regulatory expectations for consistency in drug release profiles and the growing emphasis on lifecycle management are also driving the need for specialized partners in this space.
What Makes GRDDS So Technically Demanding - And Attractive to Outsource?
Gastroretentive drug delivery systems are inherently complex, involving advanced technologies that must ensure precise control over drug release kinetics, buoyancy, adhesion, or expansion in the stomach. These requirements place significant demands on formulation design, choice of excipients, and real-time in vitro/in vivo correlation testing. Small and mid-sized pharmaceutical companies often lack the in-house infrastructure to develop such systems independently, especially for early-phase development. Outsourcing allows them access to advanced analytical tools, GRDDS-specific excipient libraries, and pilot-scale production facilities without the capital burden.Another major factor is the growing interest in polymer science, where novel bioadhesive polymers and hydrogels are being developed to improve gastric retention and drug absorption. CDMOs with expertise in material science and controlled-release platforms are now critical collaborators in accelerating time-to-market for GRDDS-enabled drugs. Additionally, the increasing demand for oral biologics and peptides, which often degrade in the intestines and benefit from upper GI absorption, is pushing formulators to seek GRDDS routes. This niche capability is rarely available in-house for many pharmaceutical firms, positioning outsourcing partners as key enablers in addressing these emerging formulation challenges.
Which Therapeutic Areas and End-Use Trends Are Shaping the Demand?
Therapeutic applications of GRDDS are expanding beyond the traditional focus areas of gastrointestinal health. While peptic ulcers, Helicobacter pylori eradication, and gastroesophageal reflux disease (GERD) still dominate usage, there is a marked rise in the application of GRDDS in the management of metabolic disorders, Parkinson’ s disease, and infectious diseases requiring site-specific drug delivery in the stomach. Drugs with pH-dependent solubility or poor stability in intestinal fluids are particularly well-suited to GRDDS, making this approach attractive for reformulation strategies and lifecycle extension of existing molecules.Outsourcing demand is also being driven by pharmaceutical companies aiming to serve aging populations and improve patient adherence. GRDDS technologies enable reduced dosing frequency and enhanced pharmacokinetic profiles, which are essential in chronic therapies. In pediatric and geriatric drug delivery, floating and mucoadhesive GRDDS offer better control over drug release and residence time, addressing swallowability and dose uniformity concerns. Outsourcing companies with a strong background in user-friendly oral dosage forms and regulatory dossier support for these specific populations are being actively sought out to lead development projects.
What’ s Fueling the Growth in GRDDS Outsourcing Across the Globe?
The growth in the gastroretentive drug delivery systems outsourcing market is driven by several factors, including the rising complexity of drug molecules requiring targeted delivery in the upper GI tract, the increasing prevalence of chronic gastric and metabolic diseases, and a growing pipeline of drugs that benefit from enhanced bioavailability. The evolving pharmaceutical R&D landscape - with heightened focus on lifecycle management and fixed-dose combinations - has created a fertile ground for GRDDS technologies. Outsourcing enables companies to tap into specialized knowledge bases, proprietary delivery platforms, and faster scale-up processes offered by CDMOs.Technological innovations such as smart polymers, gas-generating systems for floating formulations, and advanced 3D printing of gastroretentive tablets are further enhancing the capabilities of outsourced development partners. Additionally, regulatory clarity around GRDDS-enabled products and a strong global emphasis on quality-by-design (QbD) are encouraging early engagement with expert outsourcing providers. The expansion of CDMO services in emerging markets like India and Eastern Europe - where cost advantages are complemented by growing scientific expertise - is also fueling the outsourcing trend. As pharmaceutical companies continue to seek agility, innovation, and speed in product development, GRDDS outsourcing is set to remain a strategic focus area across the drug development value chain.
Report Scope
The report analyzes the Gastroretentive Drug Delivery Systems Outsourcing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Dosage Form (Tablets Dosage Form, Liquid Dosage Form, Microspheres Dosage Form, Capsule Dosage Form, Other Dosage Forms).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Tablets Dosage Form segment, which is expected to reach US$617.0 Million by 2030 with a CAGR of a 3.8%. The Liquid Dosage Form segment is also set to grow at 6.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $402.5 Million in 2024, and China, forecasted to grow at an impressive 8% CAGR to reach $394.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Gastroretentive Drug Delivery Systems Outsourcing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Gastroretentive Drug Delivery Systems Outsourcing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Gastroretentive Drug Delivery Systems Outsourcing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Alcon Inc., AptarGroup, Inc., Ashland Global Holdings Inc., AstraZeneca plc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Gastroretentive Drug Delivery Systems Outsourcing market report include:
- Adare Pharma Solutions
- Aenova Group
- Almac Group
- Aptar Pharma
- Ashland Global Holdings Inc.
- Catalent, Inc.
- Colorcon, Inc.
- Convatec Group
- CordenPharma International
- Evonik Industries AG
- Evotec SE
- Halozyme Therapeutics
- Lonza Group AG
- Patheon (Thermo Fisher Scientific)
- Pharmaron Beijing Co., Ltd.
- Quotient Sciences
- Recipharm AB
- Rubicon Research Pvt. Ltd.
- Siegfried Holding AG
- Vectura Group Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Adare Pharma Solutions
- Aenova Group
- Almac Group
- Aptar Pharma
- Ashland Global Holdings Inc.
- Catalent, Inc.
- Colorcon, Inc.
- Convatec Group
- CordenPharma International
- Evonik Industries AG
- Evotec SE
- Halozyme Therapeutics
- Lonza Group AG
- Patheon (Thermo Fisher Scientific)
- Pharmaron Beijing Co., Ltd.
- Quotient Sciences
- Recipharm AB
- Rubicon Research Pvt. Ltd.
- Siegfried Holding AG
- Vectura Group Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
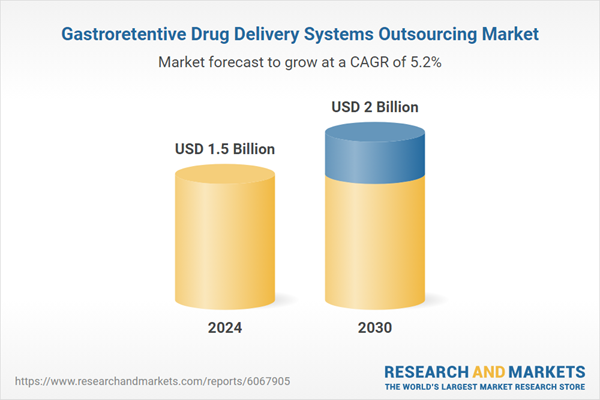
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 2 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |