Global Bone Screw Systems Market - Key Trends & Drivers Summarized
What Are the Technological Breakthroughs Revolutionizing Bone Screw Systems?
Bone screw systems are undergoing a technological revolution as advances in materials science, design innovation, and surgical integration redefine orthopedic fixation methods. The development of bioabsorbable polymers and titanium alloys has significantly enhanced the strength-to-weight ratio and biocompatibility of bone screws, reducing the risk of adverse reactions while improving osseointegration. Precision engineering and computer-aided design (CAD) tools are enabling the creation of screws with optimized thread designs, variable pitch, and self-tapping features that improve anchorage and reduce insertion torque. Innovations in surface treatments, such as plasma spraying and hydroxyapatite coatings, are promoting bone growth around the screw, thereby accelerating healing and reducing the need for removal surgeries. Digital imaging and 3D printing are being harnessed to produce patient-specific bone screw systems tailored to the anatomical contours of individual patients, thus enhancing surgical outcomes. Furthermore, the integration of smart sensor technology within screw systems is emerging, allowing real-time monitoring of load distribution and healing progress post-surgery. Advances in minimally invasive surgical techniques and robotic-assisted surgery have further refined the application of bone screw systems, reducing surgical trauma and recovery time. These technological breakthroughs are not only elevating clinical performance but also broadening the range of indications for which bone screws can be safely and effectively utilized in trauma, spinal, and reconstructive surgeries. Continuous research and development in this field are driving improvements in both design and functionality, ensuring that bone screw systems remain at the forefront of orthopedic innovation and patient care.How Are End-Use Applications and Clinical Demands Shaping the Bone Screw Systems Market?
The adoption of bone screw systems is expanding rapidly across a range of clinical applications, driven by evolving surgical techniques and increasing patient expectations for rapid recovery and long-term stability. In trauma and fracture management, bone screws play a critical role in achieving precise alignment and stable fixation, which are essential for effective bone healing. Spinal surgeries have seen significant advancements with the use of specialized pedicle screws designed to accommodate complex anatomical structures while providing robust support. The field of reconstructive and corrective orthopedic surgery is increasingly relying on bone screw systems to secure prosthetics and to enable the integration of advanced materials in joint replacement procedures. Customized screw designs and patient-specific implants are being developed to meet the unique challenges presented by conditions such as osteoporosis, where bone quality is compromised. The trend toward minimally invasive surgical techniques has also spurred demand for bone screws that are compatible with percutaneous instrumentation, thereby reducing incision size and postoperative recovery time. Surgeons are now leveraging digital preoperative planning and intraoperative navigation to optimize screw placement, which improves accuracy and reduces complications. These evolving clinical demands, combined with an increasing focus on patient outcomes and surgical efficiency, are reshaping the landscape of the bone screw systems market. Hospitals and specialized orthopedic centers are increasingly investing in the latest fixation technologies to offer state-of-the-art care, while global trends in healthcare digitization are further facilitating the adoption of advanced bone screw systems in diverse surgical environments.What Are the Market Dynamics and Regulatory Trends Influencing Bone Screw Systems?
The bone screw systems market is strongly influenced by a complex interplay of regulatory standards, market dynamics, and technological progress that shape product development and clinical adoption. Regulatory bodies such as the U.S. FDA, European CE, and other international agencies impose strict guidelines regarding the biocompatibility, mechanical performance, and sterilization processes of bone screw systems, driving manufacturers to invest in comprehensive clinical testing and quality assurance protocols. These standards ensure that new screw designs meet rigorous safety and efficacy benchmarks, which in turn build trust among surgeons and patients. Market dynamics are further influenced by the growing demand for minimally invasive procedures, personalized implants, and bioactive coatings, prompting continuous innovation in product design and material composition. Increased investments in R&D and strategic partnerships between medical device companies and research institutions are accelerating the development of next-generation bone screw systems. Global supply chain considerations, including the availability of high-grade raw materials and the adoption of precision manufacturing techniques, are also critical factors shaping market trends. Moreover, healthcare providers are increasingly emphasizing value-based care and patient outcomes, which drives the need for fixation devices that not only provide immediate stability but also promote long-term bone healing. These regulatory and market forces are creating a competitive landscape where innovation, safety, and clinical performance are paramount, influencing both product design and market positioning across the orthopedic sector.The Growth in the Bone Screw Systems Market Is Driven by Several Factors…
The growth in the bone screw systems market is driven by several factors, including breakthroughs in biocompatible materials and design optimization, an expanding range of clinical applications, and robust regulatory frameworks that ensure safety and efficacy. Technological innovations such as patient-specific implants, smart bone screws with embedded sensors, and advanced surface treatments are enhancing fixation reliability and accelerating bone healing. The rising prevalence of orthopedic conditions, combined with an aging global population and increased trauma cases, is significantly expanding the clinical need for durable, high-performance fixation systems. Additionally, the shift toward minimally invasive surgical techniques and digital surgical planning is driving demand for bone screws that offer precision and adaptability across various anatomical challenges. Regulatory approvals and international quality standards are providing a stable foundation for market growth, while strategic collaborations between device manufacturers and healthcare providers are fostering innovation and driving market expansion. These factors, together with continuous investments in R&D and a strong focus on improving patient outcomes, are propelling the bone screw systems market toward sustained global growth and heightened clinical adoption.Report Scope
The report analyzes the Bone Screw Systems market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Material Type (Stainless Steel screws, Titanium Alloy Screws, Bioabsorbable Screws); Screw Type (Cannulated Screws, Cortex Screws, Cancellous Bone Screws, Lag Screws, Headless Compression Screws); Application (Lower Extremity Application, Spinal Fusion Application, Upper Extremity Application, Other Applications); End-Use (Hospitals End-Use, Orthopedic Clinics End-Use, Ambulatory Surgical Centers End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Stainless Steel Screws segment, which is expected to reach US$1.2 Billion by 2030 with a CAGR of a 5%. The Titanium Alloy Screws segment is also set to grow at 3.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $475.1 Million in 2024, and China, forecasted to grow at an impressive 7.9% CAGR to reach $463.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bone Screw Systems Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bone Screw Systems Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bone Screw Systems Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ananka Group, Asia Bolts Industries LLC, Birmingham Fastener and Supply Inc., Boltun Corporation, Ltd., Brunner Manufacturing Co., Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Bone Screw Systems market report include:
- Acumed LLC
- Alphatec Spine, Inc. (ATEC)
- Altimed JSC
- Arthrex, Inc.
- Biopro, Inc.
- ConMed Corporation
- DePuy Synthes
- GPC Medical Ltd.
- Integra Lifesciences Corporation
- Jeil Medical Corporation
- Medtronic PLC
- Meira Oy
- NuVasive, Inc.
- Orthofix International N.V.
- Osteogenics Biomedical, Inc.
- Smith & Nephew PLC
- Spineology
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acumed LLC
- Alphatec Spine, Inc. (ATEC)
- Altimed JSC
- Arthrex, Inc.
- Biopro, Inc.
- ConMed Corporation
- DePuy Synthes
- GPC Medical Ltd.
- Integra Lifesciences Corporation
- Jeil Medical Corporation
- Medtronic PLC
- Meira Oy
- NuVasive, Inc.
- Orthofix International N.V.
- Osteogenics Biomedical, Inc.
- Smith & Nephew PLC
- Spineology
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 479 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
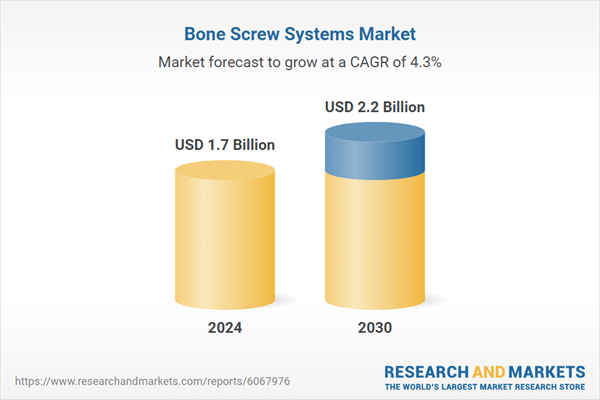
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 2.2 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |