Global Nucleic Acid Therapeutics CDMO Market - Key Trends & Drivers Summarized
Why Are Nucleic Acid Therapeutics Reshaping the Role of CDMOs in the Biopharmaceutical Supply Chain?
Nucleic acid therapeutics - comprising modalities like antisense oligonucleotides (ASOs), small interfering RNA (siRNA), messenger RNA (mRNA), aptamers, and CRISPR-based gene editors - are driving a paradigm shift in the global pharmaceutical landscape. Their growing potential to treat previously intractable diseases, from rare genetic disorders to cancer and infectious diseases, is generating a massive wave of research and commercial interest. In this context, contract development and manufacturing organizations (CDMOs) have emerged as essential partners in the delivery pipeline. Unlike traditional biologics or small molecules, nucleic acid therapeutics require highly specialized capabilities in oligonucleotide synthesis, enzymatic production, lipid nanoparticle formulation, and sterile fill-finish processes. The complexity, customization, and regulatory rigor involved in developing and scaling these therapies have prompted biopharma innovators - especially small and mid-sized biotech firms - to increasingly outsource their manufacturing operations to experienced CDMOs. As clinical pipelines grow and diversify, CDMOs are assuming more strategic roles in process optimization, analytical method development, and cGMP manufacturing, often becoming long-term collaborators from preclinical stages to commercial launch. The success of mRNA-based COVID-19 vaccines has also validated the scalability and commercial viability of nucleic acid platforms, further accelerating demand for CDMOs with robust infrastructure, technical expertise, and regulatory track records in this space.How Are Technological Capabilities and Infrastructure Investments Driving CDMO Differentiation?
With the nucleic acid therapeutics market maturing, CDMOs are undergoing a significant transformation in their service portfolios and technological capabilities to keep pace with evolving client demands. High-performance oligonucleotide synthesis platforms, automated purification systems, and next-generation analytical tools are now central to a CDMO’ s competitive edge. In parallel, the industry is seeing massive investments in dedicated manufacturing suites for RNA and DNA therapeutics, modular cleanroom facilities, and high-throughput fill-finish lines capable of handling complex formulations such as lipid nanoparticle (LNP) encapsulated mRNA. Single-use bioreactor systems, closed processing units, and microfluidic technologies are being adopted to minimize contamination risks and optimize yields.How Are Regulatory Complexity and Market Dynamics Shaping the CDMO Opportunity Landscape?
As nucleic acid therapeutics transition from experimental platforms to mainstream treatment modalities, the regulatory environment surrounding their development and manufacturing is rapidly evolving. Agencies such as the FDA, EMA, and PMDA are introducing more specific guidelines for oligonucleotide synthesis, mRNA production, and LNP formulation, which places additional compliance burdens on CDMOs. Those capable of navigating this complexity with regulatory foresight and robust quality systems are increasingly favored by biopharma clients aiming to de-risk their development paths. Moreover, the trend toward accelerated approvals, fast-track designations, and orphan drug incentives has created compressed timelines, compelling sponsors to rely more heavily on CDMOs with proven track records in rapid scale-up and tech transfer. Simultaneously, global disparities in cold-chain logistics, GMP standards, and local regulatory expectations are pushing CDMOs to diversify their operations and establish regionally compliant hubs in North America, Europe, and Asia-Pacific. The competitive intensity in the nucleic acid CDMO space is also heightened by the entry of legacy biologics manufacturers and emerging players, leading to a surge in capacity building, talent acquisition, and specialization. Additionally, pricing pressures and cost-sensitive clients in the rare disease and personalized medicine sectors are prompting CDMOs to balance customization with manufacturing efficiency. These market dynamics are not only reshaping CDMO-client relationships but are also fostering the emergence of innovation-focused, quality-driven service models across the nucleic acid therapeutics value chain.What’ s Driving the Growth of the Nucleic Acid Therapeutics CDMO Market?
The growth in the nucleic acid therapeutics CDMO market is driven by several factors spanning technological innovation, evolving biopharma pipelines, changing regulatory landscapes, and increasing outsourcing trends. First, the rapid expansion of mRNA, siRNA, ASO, and gene-editing programs across biotech and pharmaceutical companies is creating sustained demand for specialized development and manufacturing expertise. Second, the technical and logistical complexity of producing nucleic acid-based drugs - especially those requiring lipid nanoparticles, cold-chain handling, and cGMP-grade purity - is pushing sponsors to engage with CDMOs that offer end-to-end capabilities. Third, the success of mRNA vaccines and the broader validation of nucleic acid platforms have attracted significant investment into the sector, enabling both established and emerging CDMOs to expand infrastructure and innovate in process technology. Fourth, the global push toward faster development timelines and regulatory acceleration is driving demand for CDMOs that can navigate complex compliance requirements while ensuring rapid scalability. Fifth, personalized medicine, rare disease treatments, and oncology pipelines increasingly rely on nucleic acid modalities, further boosting the need for flexible, agile manufacturing partners. In addition, rising outsourcing across the biopharma sector - particularly by small and virtual biotech firms with limited in-house capacity - is fueling continuous demand for CDMO collaboration. Together, these factors are shaping a robust, high-growth global market for CDMOs specializing in nucleic acid therapeutics, positioning them as critical enablers of the next wave of biopharmaceutical innovation.Report Scope
The report analyzes the Nucleic Acid Therapeutics CDMO market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (RNA-based Therapies, Gene Therapies); Service (Manufacturing Services, Process Development & Optimization Services, Analytical & Quality Control Services, Other Services); End-Use (Biotech Companies End-Use, Pharmaceutical Companies End-Use, Government & Academic Research Institutes End-Use); Application (Genetic Disorders, Rare Diseases, Infectious Diseases, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the RNA-based Therapies segment, which is expected to reach US$28.3 Billion by 2030 with a CAGR of a 17.3%. The Gene Therapies segment is also set to grow at 11.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.4 Billion in 2024, and China, forecasted to grow at an impressive 14.6% CAGR to reach $6.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nucleic Acid Therapeutics CDMO Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nucleic Acid Therapeutics CDMO Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nucleic Acid Therapeutics CDMO Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Ltd, Curtiss-Wright Corporation, Efacec, Emerson Electric Co., Fortum and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Nucleic Acid Therapeutics CDMO market report include:
- Agilent Technologies, Inc.
- Ajinomoto Co., Inc.
- Bachem Holding AG
- BioCina Pty Ltd.
- BIOSPRING GmbH
- Catalent, Inc.
- Corden Pharma International
- Curia Global, Inc.
- Danaher Corporation (Aldevron)
- Eurogentec
- FUJIFILM Diosynth Biotechnologies
- Integrated DNA Technologies, Inc.
- KNC Laboratories Co., Ltd.
- LGC Limited
- Lonza Group Ltd
- Merck KGaA
- Nitto Denko Avecia Inc.
- Syngene International Limited
- Thermo Fisher Scientific Inc.
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Agilent Technologies, Inc.
- Ajinomoto Co., Inc.
- Bachem Holding AG
- BioCina Pty Ltd.
- BIOSPRING GmbH
- Catalent, Inc.
- Corden Pharma International
- Curia Global, Inc.
- Danaher Corporation (Aldevron)
- Eurogentec
- FUJIFILM Diosynth Biotechnologies
- Integrated DNA Technologies, Inc.
- KNC Laboratories Co., Ltd.
- LGC Limited
- Lonza Group Ltd
- Merck KGaA
- Nitto Denko Avecia Inc.
- Syngene International Limited
- Thermo Fisher Scientific Inc.
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
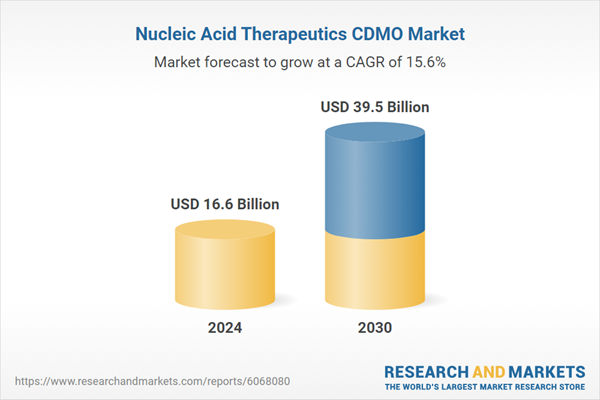
| Estimated Market Value ( USD | $ 16.6 Billion |
| Forecasted Market Value ( USD | $ 39.5 Billion |
| Compound Annual Growth Rate | 15.6% |
| Regions Covered | Global |