Global Epilepsy Monitoring Devices Market - Key Trends & Drivers Summarized
How Is Technology Transforming Epilepsy Monitoring?
The epilepsy monitoring devices market is witnessing rapid advancements as wearable sensors, artificial intelligence (AI), and remote monitoring technologies enhance patient care. Epilepsy, a neurological disorder characterized by recurrent seizures, requires continuous monitoring to improve diagnosis, treatment, and management. Traditional electroencephalogram (EEG) monitoring is being supplemented by innovative wearable and implantable devices that provide real-time seizure detection and data analytics. These devices offer improved accuracy in tracking seizure patterns, helping physicians tailor treatment plans for individual patients.The integration of AI-driven analytics is revolutionizing epilepsy monitoring by enabling predictive modeling and automated seizure detection. Machine learning algorithms can analyze large datasets from EEG recordings, wearables, and mobile applications, allowing for early intervention and personalized treatment. Additionally, remote monitoring solutions are expanding access to epilepsy care, particularly for patients in rural or underserved regions. With healthcare shifting toward digital and home-based care models, epilepsy monitoring devices are becoming more sophisticated and accessible.
What Are the Key Innovations in Epilepsy Monitoring Devices?
Recent technological breakthroughs are enhancing the accuracy, portability, and functionality of epilepsy monitoring devices. Wearable seizure detection devices, including smartwatches and biosensors, are gaining popularity for their ability to provide continuous monitoring without disrupting daily activities. Implantable neurostimulators, such as vagus nerve stimulators (VNS) and responsive neurostimulation (RNS) systems, are being used to prevent seizures in drug-resistant epilepsy patients. These advanced devices detect abnormal brain activity and deliver targeted stimulation to prevent or reduce seizure severity.Cloud-based platforms and mobile applications are also playing a crucial role in epilepsy management, allowing patients to log seizure occurrences, medication adherence, and triggers. AI-powered analytics enhance data interpretation, providing valuable insights to healthcare providers for optimizing treatment strategies. As epilepsy research advances and patient-centric solutions become more prevalent, monitoring devices will continue to evolve, improving quality of life for individuals with epilepsy.
What Are the Key Factors Driving Growth in the Epilepsy Monitoring Devices Market?
The growth in the epilepsy monitoring devices market is driven by increasing epilepsy prevalence, technological advancements in wearable and implantable monitoring solutions, and the rising adoption of remote patient monitoring. AI-driven analytics and predictive seizure detection are enhancing diagnosis accuracy and treatment planning. The growing demand for non-invasive, user-friendly monitoring solutions is further accelerating market expansion.Government initiatives and healthcare funding for neurological disorder research are contributing to increased access to epilepsy monitoring technologies. The expansion of telemedicine and mobile health (mHealth) applications is also improving epilepsy care by enabling real-time patient tracking and physician-patient communication. As the focus on personalized medicine and digital health intensifies, the epilepsy monitoring devices market is set for continued innovation and growth.
Report Scope
The report analyzes the Epilepsy Monitoring Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Wearable Devices, Conventional Devices); End-Use (Hospitals & Clinics, Ambulatory Surgery Centers, Neurology Centers, Diagnostic Centers).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Wearable Devices segment, which is expected to reach US$1.1 Billion by 2030 with a CAGR of a 5.4%. The Conventional Devices segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $315.0 Million in 2024, and China, forecasted to grow at an impressive 8.4% CAGR to reach $315.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Epilepsy Monitoring Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Epilepsy Monitoring Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Epilepsy Monitoring Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aker BioMarine AS, Algarithm Ingredients, Archer-Daniels-Midland Company, Arista Industries Inc., BASF SE and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Epilepsy Monitoring Devices market report include:
- BioSerenity
- Boston Scientific Corporation
- Cadwell Industries, Inc.
- Ceribell, Inc.
- Compumedics Limited
- Drägerwerk AG & Co. KGaA
- Emfit Ltd.
- Empatica Inc.
- Koninklijke Philips N.V.
- Lifelines Neuro
- Magstim
- Masimo Corporation
- Medtronic plc
- Natus Medical Incorporated
- NeuroPace, Inc.
- Neurosoft
- NeuroWave Systems Inc.
- Nihon Kohden Corporation
- Nonin Medical, Inc.
- Seer Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- BioSerenity
- Boston Scientific Corporation
- Cadwell Industries, Inc.
- Ceribell, Inc.
- Compumedics Limited
- Drägerwerk AG & Co. KGaA
- Emfit Ltd.
- Empatica Inc.
- Koninklijke Philips N.V.
- Lifelines Neuro
- Magstim
- Masimo Corporation
- Medtronic plc
- Natus Medical Incorporated
- NeuroPace, Inc.
- Neurosoft
- NeuroWave Systems Inc.
- Nihon Kohden Corporation
- Nonin Medical, Inc.
- Seer Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 271 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
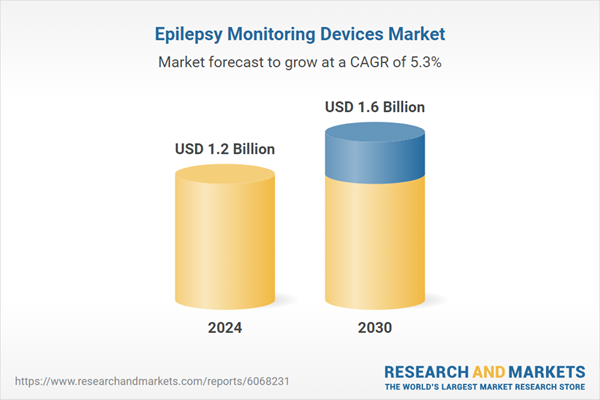
| Estimated Market Value ( USD | $ 1.2 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |