Global Rare Disease Clinical Trials Market - Key Trends & Drivers Summarized
Why Are Rare Disease Clinical Trials Gaining Importance in Drug Development?
Rare disease clinical trials have become a focal point in drug development as pharmaceutical companies and regulatory agencies intensify efforts to address the unmet medical needs of patients suffering from rare and orphan diseases. With over 7,000 identified rare diseases affecting millions worldwide, the demand for effective therapies has grown exponentially. Traditional drug development models often overlook rare diseases due to limited patient populations and high research costs, but advancements in genomics, targeted therapies, and regulatory incentives have transformed the landscape. Governments and health organizations are providing grants, tax credits, and accelerated approval pathways to encourage rare disease drug development. The increasing adoption of adaptive trial designs, patient-centric approaches, and decentralized clinical trials has further improved the feasibility of conducting research on rare diseases. As the need for novel treatments rises, rare disease clinical trials are becoming an integral part of biopharmaceutical innovation.How Are Emerging Technologies Improving Rare Disease Clinical Trials?
Technological advancements have significantly enhanced the efficiency and success rates of rare disease clinical trials. Artificial intelligence (AI) and machine learning are streamlining patient recruitment by identifying eligible participants based on genetic profiles and electronic health records (EHRs). The use of real-world data (RWD) and real-world evidence (RWE) is helping researchers gain insights into disease progression and treatment effectiveness, reducing the reliance on large patient cohorts. The adoption of virtual clinical trials and remote patient monitoring has improved trial accessibility, particularly for geographically dispersed rare disease patients. Biomarker-driven drug development and precision medicine approaches have also accelerated rare disease research, allowing for more targeted and effective treatment strategies. Additionally, gene therapy and CRISPR-based technologies are paving the way for groundbreaking rare disease treatments, reducing trial timelines and increasing the likelihood of successful outcomes.What Market Trends Are Driving Rare Disease Clinical Trial Expansion?
The growing focus on orphan drug development and regulatory incentives has significantly boosted the rare disease clinical trial market. The FDA's Orphan Drug Act and the European Medicines Agency's (EMA) orphan designation program have incentivized pharmaceutical companies to invest in rare disease research by offering market exclusivity, reduced fees, and priority review programs. The rise of patient advocacy groups and increased patient engagement in trial designs have improved trial retention rates and recruitment efficiency. The expansion of gene and cell therapy research has led to a surge in clinical trials targeting rare genetic disorders, rare cancers, and neurodegenerative diseases. Additionally, strategic collaborations between pharmaceutical companies, biotech firms, and research institutions are driving innovation in rare disease drug development. The emergence of global rare disease registries and AI-powered clinical trial platforms is further enhancing trial design and data analysis, making rare disease research more viable and impactful.What Are the Key Growth Drivers of the Rare Disease Clinical Trials Market?
The growth in the global rare disease clinical trials market is driven by several factors, including increasing regulatory support, advancements in genomic medicine, and the expansion of decentralized clinical trial models. The rising incidence of rare genetic disorders and the demand for personalized treatments have intensified the need for efficient and targeted clinical trials. The integration of AI-driven analytics, virtual patient monitoring, and real-world data is optimizing trial processes, reducing costs, and accelerating drug approvals. Additionally, biopharmaceutical companies are leveraging patient-centric trial methodologies to improve recruitment and retention rates, ensuring the success of rare disease studies. The growing investment in orphan drug development, coupled with collaborative efforts between pharmaceutical companies and patient advocacy groups, is further fueling market expansion. As rare disease research continues to evolve, innovative trial designs and cutting-edge biotechnologies are expected to drive breakthroughs in treatment development, improving outcomes for patients worldwide.Report Scope
The report analyzes the Rare Disease Clinical Trials market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Phase (Phase I, Phase II, Phase III, Phase IV); Therapeutic Area (Oncology, Cardiovascular Disorders, Neurological Disorders, Infectious Diseases, Genetic Disorders, Autoimmune & Inflammation, Hematologic Disorders, Musculoskeletal Disorders, Other Therapeutic Areas); Sponsor (Pharma & Biotech Companies, Non-Profit Organizations, Other Sponsors).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Some of the 37 companies featured in this Rare Disease Clinical Trials market report include -
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Amgen Inc.
- Arcturus Therapeutics
- AstraZeneca
- Biogen Inc.
- Charles River Laboratories
- Editas Medicine
- F. Hoffmann-La Roche Ltd.
- Fortrea
- ICON plc
- IQVIA Inc.
- Labcorp Drug Development
- Medpace
- Novartis AG
- Parexel International Corporation
- Pfizer Inc.
- Precision for Medicine
- PTC Therapeutics
- Sanofi
- Scholar Rock
- Takeda Pharmaceutical Company
- Ultragenyx Pharmaceutical Inc.
- United Therapeutics
- Vertex Pharmaceuticals
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$8.6 Billion by 2030 with a CAGR of a 7.5%. The Phase II Clinical Trials segment is also set to grow at 4.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.7 Billion in 2024, and China, forecasted to grow at an impressive 5.7% CAGR to reach $3.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Rare Disease Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Rare Disease Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Rare Disease Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Akadeum Life Sciences, Inc., Applied Cells, Beckman Coulter, Inc., Becton, Dickinson and Company, BioFluidica and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 37 Featured):
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Amgen Inc.
- Arcturus Therapeutics
- AstraZeneca
- Biogen Inc.
- Charles River Laboratories
- Editas Medicine
- F. Hoffmann-La Roche Ltd.
- Fortrea
- ICON plc
- IQVIA Inc.
- Labcorp Drug Development
- Medpace
- Novartis AG
- Parexel International Corporation
- Pfizer Inc.
- Precision for Medicine
- PTC Therapeutics
- Sanofi
- Scholar Rock
- Takeda Pharmaceutical Company
- Ultragenyx Pharmaceutical Inc.
- United Therapeutics
- Vertex Pharmaceuticals
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Amgen Inc.
- Arcturus Therapeutics
- AstraZeneca
- Biogen Inc.
- Charles River Laboratories
- Editas Medicine
- F. Hoffmann-La Roche Ltd.
- Fortrea
- ICON plc
- IQVIA Inc.
- Labcorp Drug Development
- Medpace
- Novartis AG
- Parexel International Corporation
- Pfizer Inc.
- Precision for Medicine
- PTC Therapeutics
- Sanofi
- Scholar Rock
- Takeda Pharmaceutical Company
- Ultragenyx Pharmaceutical Inc.
- United Therapeutics
- Vertex Pharmaceuticals
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
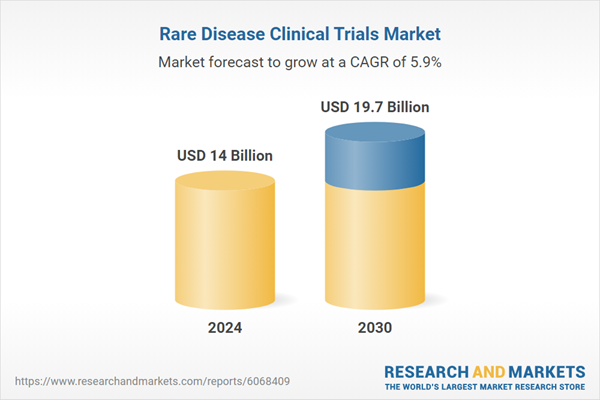
| Estimated Market Value ( USD | $ 14 Billion |
| Forecasted Market Value ( USD | $ 19.7 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |