Global Non-Invasive Brain Trauma Monitoring Devices Market - Key Trends & Drivers Summarized
How Are Non-Invasive Brain Monitoring Devices Transforming Neuro-Critical Care?
The global surge in the demand for non-invasive brain trauma monitoring devices represents a transformative shift in how neuro-critical care is delivered and accessed. Traditionally, monitoring brain trauma involved invasive procedures such as inserting catheters or conducting craniotomies to measure intracranial pressure (ICP) and other cerebral parameters. These invasive approaches, while effective, often posed considerable risks including infections, bleeding, and extended hospital stays. Today, with advancements in medical device engineering and neurotechnology, non-invasive alternatives are offering a much safer and patient-friendly path forward. Technologies such as near-infrared spectroscopy (NIRS), transcranial Doppler (TCD) ultrasound, and quantitative electroencephalography (qEEG) have enabled clinicians to monitor critical brain functions without breaching the skull, allowing for real-time, continuous observation of cerebral dynamics. These innovations are particularly valuable in emergency medicine, military operations, and rural healthcare settings where time and resources are limited. In intensive care units, non-invasive monitoring helps reduce intervention delays while supporting more precise and tailored care pathways. They are now frequently used alongside other vital sign monitoring tools to develop a comprehensive neurological picture, aiding in early detection of secondary brain injuries such as swelling or hypoxia. Furthermore, with the increasing availability of portable and bedside-compatible units, these devices are being adopted in ambulances, field hospitals, and even on sports sidelines. The non-invasive approach significantly enhances patient comfort and lowers procedure-associated costs, encouraging widespread institutional adoption. As clinical outcomes continue to favor less intrusive methods, these technologies are fast becoming essential components in standard trauma protocols and neurological evaluations worldwide.Can Non-Invasive Devices Bridge the Gap in Early Diagnosis and Intervention?
Early and precise diagnosis of traumatic brain injury (TBI) is a decisive factor in preventing long-term neurological damage and enhancing recovery prospects. However, in many cases, subtle symptoms of brain trauma go unnoticed until they evolve into severe complications, particularly when conventional imaging and monitoring tools are unavailable or impractical. Non-invasive brain trauma monitoring devices are increasingly stepping into this diagnostic void by providing rapid, accessible, and continuous cerebral assessment across various clinical and non-clinical environments. Tools such as functional NIRS and advanced EEG systems can track changes in cerebral blood oxygenation, electrical activity, and intracranial dynamics in real time, without requiring surgical intervention. This is a game-changer in environments like sports fields, battlefield settings, and emergency response units, where immediate diagnostics can shape the course of treatment. In pediatrics, non-invasive monitoring is proving particularly beneficial, enabling physicians to assess head injuries in children without subjecting them to the risks associated with CT scans or sedation. Additionally, for patients with mild TBIs or concussions who may not require hospitalization, these devices offer a valuable means of outpatient monitoring, thereby reducing pressure on hospital resources. Importantly, their use is expanding into telemedicine applications, where data from wearable or portable non-invasive monitors can be transmitted to neurologists remotely for interpretation. This capability opens doors to improved care access in underserved or geographically isolated communities. Moreover, the integration of continuous monitoring with early intervention strategies is helping mitigate secondary injuries caused by delayed treatment, significantly improving clinical outcomes. By facilitating fast and non-disruptive assessments, these devices are enabling earlier therapeutic decisions, reducing morbidity, and preventing the escalation of trauma-related complications.How Is Innovation in Imaging and AI Rewriting the Monitoring Paradigm?
The non-invasive brain trauma monitoring landscape is experiencing rapid evolution, driven largely by innovations in imaging, miniaturization, and artificial intelligence (AI). One of the most transformative changes is the integration of AI algorithms capable of analyzing vast amounts of neurological data in real time to detect early signs of cerebral compromise. These algorithms can process complex patterns from EEG waveforms, hemodynamic signals, and brain perfusion metrics to provide clinicians with actionable insights faster than ever before. Additionally, hybrid monitoring systems that combine multiple non-invasive modalities - such as EEG with NIRS or Doppler ultrasound - are becoming more common. These multi-parametric platforms offer a more holistic view of brain activity and function, enhancing diagnostic accuracy. Miniaturized sensors and wireless capabilities are also enabling continuous and ambulatory monitoring, a feature especially useful in rehabilitation settings or for long-term concussion management in sports. Innovations in 3D brain imaging and photonic sensors are pushing boundaries even further, allowing clinicians to detect microvascular abnormalities and monitor localized brain metabolism non-invasively. Cloud-based data platforms are also being employed to store patient data across timeframes, facilitating longitudinal studies, predictive analytics, and outcome-based tracking. The result is not just more accurate diagnostics, but also the ability to personalize treatment based on individual cerebral profiles. In parallel, the user interface for these devices is becoming more clinician-friendly, often integrating with hospital EHR systems and providing visual cues or alerts based on pre-programmed thresholds. Regulatory bodies are starting to recognize the value of such technologies, speeding up approvals and fostering competition. Together, these innovations are redefining the neurological monitoring paradigm, positioning non-invasive tools not just as alternatives to invasive methods, but as superior diagnostic solutions in many clinical contexts.What Factors Are Fueling the Growth of the Non-Invasive Brain Monitoring Market?
The growth in the non-invasive brain trauma monitoring devices market is driven by several factors spanning technological breakthroughs, clinical need, changing healthcare models, and evolving patient expectations. One of the key drivers is the significant advancement in sensor technology and digital signal processing, which has dramatically improved the sensitivity, specificity, and reliability of non-invasive brain monitoring systems. These improvements are making non-invasive tools viable for critical applications that were once the domain of invasive procedures. Another major factor is the increasing global incidence of traumatic brain injuries resulting from vehicular accidents, sports injuries, falls in aging populations, and military combat. These demographic and situational trends are creating sustained demand for efficient, real-time cerebral monitoring solutions. Furthermore, the shift toward value-based care and outpatient services is prompting healthcare institutions to adopt technologies that reduce hospital stays, lower procedural risks, and support remote patient monitoring. Non-invasive devices align perfectly with these goals by enabling cost-effective, scalable, and patient-centric monitoring approaches. Additionally, a growing body of clinical evidence supporting the use of non-invasive tools for early detection of conditions like stroke, epilepsy, and post-surgical complications is encouraging adoption across broader medical specialties. Governments and public health organizations are also stepping in, offering funding and policy support for innovation in neuro-monitoring technologies, particularly in rural and underdeveloped healthcare systems. From a commercial standpoint, the influx of investment into neurotechnology startups and increased merger and acquisition activity in the medical device sector is accelerating the development and distribution of next-generation devices. Lastly, increasing awareness among patients and caregivers about brain health, cognitive longevity, and post-trauma care is elevating demand for accessible and non-intimidating monitoring options. Collectively, these converging trends are propelling the non-invasive brain trauma monitoring devices market forward, making it one of the most dynamic and impactful segments in neuro-diagnostics today.Report Scope
The report analyzes the Non-invasive Brain Trauma Monitoring Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Non-Invasive Brain Trauma Monitoring Devices, Non-Invasive Brain Trauma Consumables); End-Use (Hospitals End-Use, Neurological Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Non-Invasive Brain Trauma Monitoring Devices segment, which is expected to reach US$15.9 Billion by 2030 with a CAGR of a 9.2%. The Non-Invasive Brain Trauma Consumables segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.0 Billion in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $5.0 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Non-invasive Brain Trauma Monitoring Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Non-invasive Brain Trauma Monitoring Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Non-invasive Brain Trauma Monitoring Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Andechser Molkerei Scheitz GmbH, Bellwether Farms, Brown Cow (Stonyfield Farm, Inc.), Chobani LLC, Clover Sonoma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Non-invasive Brain Trauma Monitoring Devices market report include:
- Advanced Brain Monitoring, Inc.
- Brain Scientific Inc.
- BrainScope Company, Inc.
- Cerebrotech Medical Systems
- Ceribell, Inc.
- Compumedics Limited
- Elekta AB
- GE Healthcare
- Integra LifeSciences Holdings Corporation
- Kernel
- Masimo Corporation
- Medtronic plc
- Natus Medical Incorporated
- Neuroelectrics
- Nihon Kohden Corporation
- Philips Healthcare
- Precision Neuroscience
- RAUMEDIC AG
- SOPHYSA
- SPIEGELBERG GmbH & Co. KG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advanced Brain Monitoring, Inc.
- Brain Scientific Inc.
- BrainScope Company, Inc.
- Cerebrotech Medical Systems
- Ceribell, Inc.
- Compumedics Limited
- Elekta AB
- GE Healthcare
- Integra LifeSciences Holdings Corporation
- Kernel
- Masimo Corporation
- Medtronic plc
- Natus Medical Incorporated
- Neuroelectrics
- Nihon Kohden Corporation
- Philips Healthcare
- Precision Neuroscience
- RAUMEDIC AG
- SOPHYSA
- SPIEGELBERG GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 273 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
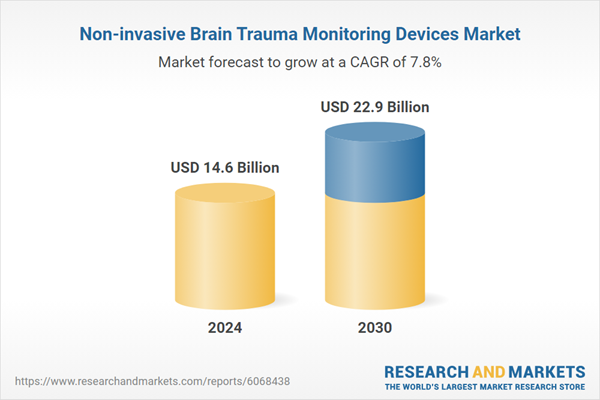
| Estimated Market Value ( USD | $ 14.6 Billion |
| Forecasted Market Value ( USD | $ 22.9 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |