Global Plasmid Purification Market - Key Trends & Drivers Summarized
The plasmid purification market is expanding rapidly, driven by the growing demand for gene therapy, mRNA vaccine production, and cell-based immunotherapies. Plasmid DNA (pDNA) is a critical component in genetic engineering, viral vector manufacturing, and CRISPR genome editing, making its purification an essential step in biopharmaceutical production. As biotech companies and research institutions seek high-purity, endotoxin-free plasmids for clinical applications, innovations in chromatography, filtration, and automated purification technologies are transforming the industry.A key trend in the market is the increasing adoption of scalable, GMP-compliant plasmid purification techniques. With gene-based medicines moving from preclinical research to large-scale commercialization, traditional alkaline lysis-based purification methods are being replaced by high-yield, automated, and regulatory-compliant processes. The rise of plasmid DNA-based vaccines, non-viral gene therapies, and recombinant protein production has driven demand for high-throughput purification platforms that ensure batch consistency, minimal contamination, and high recovery rates.
Another major driver is the growing focus on high-purity plasmid DNA for in vitro transcription (IVT) in mRNA vaccine development. The COVID-19 pandemic accelerated demand for mRNA vaccines, where plasmid DNA serves as the DNA template for IVT-based mRNA synthesis. With biotech firms investing in next-generation RNA-based therapeutics, manufacturers are optimizing plasmid purification workflows to deliver IVT-ready, endotoxin-free plasmids that meet strict regulatory and quality control standards.
Additionally, regulatory compliance and standardization in plasmid purification are shaping market dynamics. Agencies such as the FDA, EMA, and WHO are enforcing stringent GMP requirements for plasmid DNA used in gene-modified therapies, cell-based treatments, and personalized medicine. This has led to increased investment in process analytics, in-line purification monitoring, and automated endotoxin removal techniques, ensuring plasmid DNA meets purity, potency, and sterility requirements for clinical applications.
How Are Technological Innovations Transforming Plasmid Purification?
Advancements in plasmid DNA extraction, purification, and quality control are revolutionizing bioprocessing, enabling higher yields, better scalability, and reduced impurities. Traditional alkaline lysis-based extraction methods, while widely used in research settings, face scalability challenges and purity limitations, prompting the development of next-generation plasmid purification technologies.One of the most significant innovations is high-resolution chromatography-based purification techniques. Advanced anion-exchange chromatography (AEX), hydrophobic interaction chromatography (HIC), and size-exclusion chromatography (SEC) are replacing conventional precipitation and resin-based methods, offering higher recovery rates, lower endotoxin levels, and better separation of supercoiled plasmid DNA from contaminants such as genomic DNA, RNA, and host cell proteins. These chromatography platforms are essential for GMP-grade plasmid purification, ensuring plasmid integrity for gene therapy and vaccine applications.
Another major breakthrough is membrane-based and tangential flow filtration (TFF) purification, which enables scalable, high-throughput plasmid DNA isolation. TFF systems, equipped with ultrafiltration and diafiltration membranes, allow for continuous plasmid DNA concentration and buffer exchange, improving yield consistency and process efficiency. These methods minimize shear stress on plasmids, preserving their supercoiled conformation, which is crucial for high-efficiency gene transfection and therapeutic applications.
Additionally, endotoxin removal technologies have become a focal point in plasmid purification. Endotoxins, derived from bacterial cell walls, pose a significant risk in gene therapy and vaccine production, as they can trigger inflammatory responses and immune system complications. The adoption of affinity-based endotoxin removal resins, enzymatic degradation techniques, and dual-membrane filtration systems is ensuring plasmid DNA meets ultra-low endotoxin thresholds required for clinical and commercial applications.
Moreover, automation and AI-driven process control are enhancing plasmid purification efficiency. AI-powered bioprocess monitoring tools, predictive analytics, and real-time plasmid quantification systems are improving yield predictability, impurity detection, and process standardization. The integration of machine learning algorithms with chromatography and filtration platforms is optimizing flow rates, buffer compositions, and purification cycle times, reducing batch-to-batch variability and enhancing production scalability.
How Are Market Dynamics and End-Use Applications Shaping Demand?
The demand for high-quality plasmid DNA purification is being shaped by expanding applications in gene therapies, mRNA vaccines, and synthetic biology, with biopharmaceutical companies, contract manufacturers, and research institutions driving market expansion.One of the largest application segments is viral vector production for gene therapy, where plasmid DNA serves as a template for AAV, lentivirus, and adenoviral vector manufacturing. The growing adoption of CAR-T cell therapies, CRISPR-based gene editing, and non-viral gene delivery systems has increased the need for high-purity, GMP-grade plasmid DNA, pushing biomanufacturers to optimize plasmid purification efficiency and scalability.
Another key growth area is mRNA-based vaccines and therapeutics, where plasmid DNA is used as a DNA template for in vitro transcription (IVT). The surge in mRNA vaccine development for infectious diseases, oncology, and autoimmune disorders has accelerated the demand for IVT-ready plasmid DNA, requiring high-fidelity purification techniques to ensure endotoxin-free, high-yield DNA templates. Contract development and manufacturing organizations (CDMOs) are investing in scalable plasmid purification solutions to meet the increasing demand for RNA-based therapeutics.
The synthetic biology and precision medicine sectors are also driving the demand for custom plasmid purification solutions. As researchers engineer next-generation synthetic plasmids for metabolic engineering, protein synthesis, and gene circuit design, the need for high-throughput, modular plasmid purification platforms is expanding. Academic labs, biotech startups, and pharmaceutical firms require flexible, small-batch purification services, leading to the rise of custom plasmid purification workflows tailored for synthetic biology applications.
What Factors Are Driving the Growth of the Plasmid Purification Market?
The growth in the plasmid purification market is driven by several factors, including advancements in bioprocessing technology, increasing demand for high-purity plasmid DNA, scalability of GMP production, and evolving regulatory requirements. The surge in gene therapy, mRNA vaccines, and synthetic biology applications is creating unprecedented demand for ultra-pure plasmid DNA, compelling biotech firms and contract manufacturers to invest in purification process optimization.One of the primary market drivers is the expansion of biopharmaceutical R&D in genetic medicine and cell therapies. With CAR-T therapy, regenerative medicine, and genome editing platforms gaining clinical traction, the need for high-yield, contamination-free plasmid DNA is growing. This has led to the adoption of hybrid purification workflows, combining chromatography, ultrafiltration, and automated endotoxin removal technologies to meet commercial-scale plasmid manufacturing needs.
Another key factor is regulatory compliance and quality control enhancements. With FDA and EMA guidelines requiring stringent purity specifications for gene-modified therapies, plasmid manufacturers are focusing on advanced analytics, process validation, and sterility assurance to ensure plasmid DNA meets clinical-grade standards. The rise of single-use bioprocessing systems, closed-loop purification workflows, and real-time process monitoring is supporting GMP-compliant plasmid production for regulatory approvals.
Additionally, the rise of AI-driven biomanufacturing and automation is transforming plasmid purification efficiency. AI-powered chromatography control systems, predictive impurity detection, and automated buffer exchange platforms are enhancing yield reproducibility, impurity removal efficiency, and production scalability. This technological shift is making high-throughput, low-cost plasmid purification more accessible for biotech firms and CDMOs.
As gene therapy, mRNA vaccines, and synthetic biology applications continue to expand, the plasmid purification market is poised for sustained growth. Companies that invest in high-efficiency purification technologies, AI-driven process control, and regulatory-compliant manufacturing solutions will be well-positioned to lead the next phase of biopharmaceutical innovation, ensuring high-quality plasmid DNA production for the future of genetic medicine.
Report Scope
The report analyzes the Plasmid Purification market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Offering (Products, Services); Grade (Molecular Grade, Transfection Grade); Application (Cloning & Protein Expression, Transfection & Gene Editing, Others); End-Use (Pharma & Biotech Companies, Academic & Research Institutes, Contract Research Organizations).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Plasmid Purification Products segment, which is expected to reach US$2.3 Billion by 2030 with a CAGR of a 12.8%. The Plasmid Purification Services segment is also set to grow at 8.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $507.4 Million in 2024, and China, forecasted to grow at an impressive 15.4% CAGR to reach $739.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Plasmid Purification Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Plasmid Purification Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Plasmid Purification Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 53Biologics, Akron Biotech, Aldevron (Danaher Corporation), Applied Biological Materials Inc., Aurigene Pharmaceutical Services and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Plasmid Purification market report include:
- 53Biologics
- Akron Biotech
- Aldevron (Danaher Corporation)
- Applied Biological Materials Inc.
- Aurigene Pharmaceutical Services
- Biozilla
- Cobra Biologics (Cognate BioServices)
- Esco Aster PTE. LTD
- Forge Biologics
- GenScript Biotech Corporation
- Luminous BioSciences, LLC
- MACHEREY-NAGEL GmbH & Co. KG
- Nature Technology Corporation
- PlasmidFactory GmbH & Co. KG
- Promega Corporation
- SK pharmteco
- TriLink BioTechnologies
- VectorBuilder Inc.
- VGXI, Inc.
- VIVE Biotech
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 53Biologics
- Akron Biotech
- Aldevron (Danaher Corporation)
- Applied Biological Materials Inc.
- Aurigene Pharmaceutical Services
- Biozilla
- Cobra Biologics (Cognate BioServices)
- Esco Aster PTE. LTD
- Forge Biologics
- GenScript Biotech Corporation
- Luminous BioSciences, LLC
- MACHEREY-NAGEL GmbH & Co. KG
- Nature Technology Corporation
- PlasmidFactory GmbH & Co. KG
- Promega Corporation
- SK pharmteco
- TriLink BioTechnologies
- VectorBuilder Inc.
- VGXI, Inc.
- VIVE Biotech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 471 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
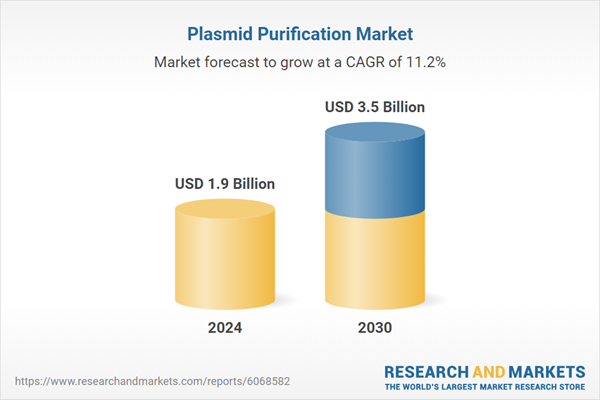
| Estimated Market Value ( USD | $ 1.9 Billion |
| Forecasted Market Value ( USD | $ 3.5 Billion |
| Compound Annual Growth Rate | 11.2% |
| Regions Covered | Global |