Global Blood Cancer Diagnostics Market - Key Trends & Drivers Summarized
Why Is Blood Cancer Diagnostics Becoming a Critical Focus in Oncology?
Blood cancer diagnostics have become an essential component of modern oncology, enabling early detection, disease classification, and personalized treatment planning for hematologic malignancies such as leukemia, lymphoma, and multiple myeloma. As the global burden of blood cancers continues to rise, healthcare providers are prioritizing advancements in diagnostic technologies to improve survival rates and treatment outcomes. Blood cancers account for a significant portion of cancer-related deaths worldwide, making early and accurate detection crucial for timely intervention.With the emergence of precision medicine and targeted therapies, the demand for high-sensitivity diagnostic tools has surged. Traditional diagnostic methods, including complete blood counts (CBC), bone marrow biopsies, and flow cytometry, are now being supplemented with advanced molecular and genetic testing. The integration of next-generation sequencing (NGS), liquid biopsy, and artificial intelligence (AI)-driven diagnostic algorithms is revolutionizing blood cancer detection, allowing for earlier diagnosis and more personalized treatment strategies. As the healthcare industry moves toward non-invasive, highly accurate diagnostic solutions, the market for blood cancer diagnostics is expected to grow rapidly.
How Are Technological Advancements Transforming Blood Cancer Diagnostics?
Significant advancements in molecular diagnostics, bioinformatics, and digital pathology are reshaping the landscape of blood cancer diagnostics, enabling faster, more precise, and less invasive detection methods. One of the most transformative innovations in this space is liquid biopsy, which allows for the detection of circulating tumor DNA (ctDNA) and other biomarkers in blood samples. Unlike traditional bone marrow biopsies, liquid biopsy offers a non-invasive alternative that can detect minimal residual disease (MRD) and monitor treatment response in real-time. This technology is particularly beneficial for patients with leukemia and lymphoma, where disease progression requires continuous monitoring.Another major breakthrough in blood cancer diagnostics is the widespread adoption of next-generation sequencing (NGS) and polymerase chain reaction (PCR)-based techniques. These molecular tools enable comprehensive genetic profiling, allowing oncologists to identify specific mutations and genetic aberrations associated with different types of blood cancer. AI-powered diagnostic algorithms are also enhancing the accuracy of hematopathology analysis, automating image recognition in blood smears and bone marrow aspirates to detect abnormal cell morphology with high precision. Furthermore, advancements in flow cytometry and immunophenotyping are improving the ability to distinguish between different subtypes of leukemia and lymphoma, facilitating more targeted treatment approaches. As these technological innovations continue to evolve, they are expected to improve early diagnosis, reduce misdiagnosis rates, and enhance overall patient management in hematologic oncology.
Which Market Trends Are Driving Growth in Blood Cancer Diagnostics?
The growing adoption of personalized medicine and targeted therapies is one of the most significant trends fueling the demand for advanced blood cancer diagnostics. With an increasing number of cancer treatments being designed to target specific genetic mutations, comprehensive molecular testing has become a critical step in treatment decision-making. This trend has led to the rapid expansion of companion diagnostics, where blood cancer patients undergo genetic testing to determine their eligibility for targeted therapies, such as tyrosine kinase inhibitors (TKIs) and monoclonal antibodies.Another key trend shaping the market is the increasing reliance on artificial intelligence and automation in cancer diagnostics. AI-powered image analysis tools are streamlining hematopathology workflows, reducing the time required for diagnosis while improving accuracy. Additionally, the expansion of decentralized testing and point-of-care diagnostics is making blood cancer detection more accessible, particularly in regions with limited healthcare infrastructure. The integration of cloud-based diagnostic platforms and telemedicine solutions is further enhancing remote diagnosis and patient monitoring, allowing oncologists to track disease progression more efficiently. As the global healthcare landscape shifts toward early cancer detection and preventive screening, the demand for innovative blood cancer diagnostics is expected to rise.
What Are the Key Growth Drivers Shaping the Future of the Blood Cancer Diagnostics Market?
The growth in the blood cancer diagnostics market is driven by multiple factors, including rising cancer prevalence, advancements in diagnostic technology, and increasing investments in precision oncology. One of the most significant drivers is the growing incidence of hematologic malignancies, which has led to a higher demand for early and accurate diagnostic solutions. According to the Leukemia & Lymphoma Society, blood cancers account for nearly 10% of all cancer diagnoses worldwide, underscoring the need for robust diagnostic infrastructure to improve detection and patient outcomes.Another crucial driver shaping the market is the increasing focus on liquid biopsy and minimally invasive diagnostic techniques. With healthcare providers and patients seeking alternatives to traditional invasive procedures, liquid biopsy has emerged as a game-changer in blood cancer detection and monitoring. Additionally, government initiatives and funding for cancer research are accelerating the development of novel diagnostic platforms, further expanding market opportunities. The rise of biomarker-driven clinical trials and regulatory approvals for advanced diagnostics is also propelling growth, as pharmaceutical companies collaborate with diagnostic firms to develop companion tests for emerging therapies.
As the field of hematologic oncology continues to advance, the integration of AI, big data analytics, and molecular diagnostics will play a pivotal role in shaping the future of blood cancer diagnostics. The increasing availability of precision diagnostics, coupled with ongoing innovations in early detection and treatment monitoring, will drive significant growth in this market, ultimately improving survival rates and quality of life for blood cancer patients worldwide.
Report Scope
The report analyzes the Blood Cancer Diagnostics market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (Instruments, Assay Kits and Reagents); Test Type (Blood Tests, Imaging Tests, Biopsy, Molecular Test); End-Use (Hospitals and Clinics End-Use, Diagnostic Labs End-Use, Research Institutes End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Instruments segment, which is expected to reach US$8.4 Billion by 2030 with a CAGR of a 6.6%. The Assay Kits and Reagents segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.9 Billion in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $2.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Blood Cancer Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Blood Cancer Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Blood Cancer Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as American Association of Blood Banks (AABB)?, American Red Cross?, Australian Red Cross?, Baxter International Inc.?, Beckman Coulter, Inc.? and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Blood Cancer Diagnostics market report include:
- Abbott Laboratories
- Adaptive Biotechnologies
- Agilent Technologies, Inc.
- Alercell, Inc.
- Asuragen (a Bio-Techne brand)
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd
- Foundation Medicine, Inc.
- GE HealthCare Technologies Inc.
- Grail, Inc.
- Guardant Health, Inc.
- HORIBA, Ltd.
- Illumina, Inc.
- InVivoScribe, Inc.
- QIAGEN N.V.
- Quest Diagnostics
- SkylineDx
- Sophia Genetics
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Adaptive Biotechnologies
- Agilent Technologies, Inc.
- Alercell, Inc.
- Asuragen (a Bio-Techne brand)
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd
- Foundation Medicine, Inc.
- GE HealthCare Technologies Inc.
- Grail, Inc.
- Guardant Health, Inc.
- HORIBA, Ltd.
- Illumina, Inc.
- InVivoScribe, Inc.
- QIAGEN N.V.
- Quest Diagnostics
- SkylineDx
- Sophia Genetics
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
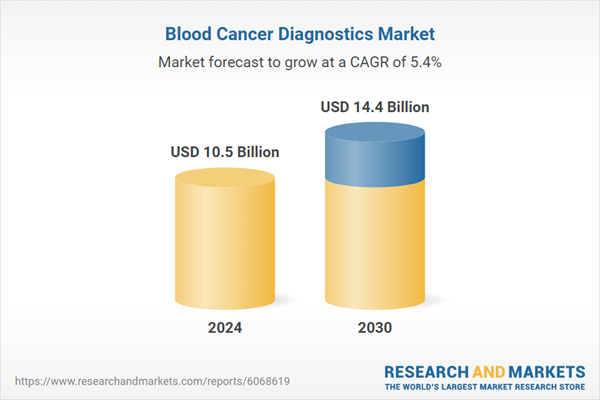
| Estimated Market Value ( USD | $ 10.5 Billion |
| Forecasted Market Value ( USD | $ 14.4 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |