Global Monkeypox Vaccine and Treatment Market - Key Trends & Drivers Summarized
Why Has the Demand for Monkeypox Vaccines and Treatments Increased Worldwide?
The resurgence of monkeypox in multiple regions across the globe has led to a significant rise in demand for effective vaccines and treatments. Previously considered a rare zoonotic disease confined to Central and West Africa, monkeypox has now spread to numerous countries, prompting the World Health Organization (WHO) and public health agencies to classify it as a growing global health threat. The emergence of human-to-human transmission in urban settings has intensified the urgency for immunization campaigns and antiviral treatments to curb outbreaks. Governments and health organizations have increased funding for vaccine procurement and distribution, particularly for high-risk populations such as immunocompromised individuals, healthcare workers, and those exposed to infected individuals. The need for long-term immunity against potential future outbreaks has also accelerated research into next-generation vaccines with improved efficacy and longer-lasting protection.What Scientific Advancements Are Shaping the Development of Monkeypox Vaccines and Treatments?
The development of vaccines and therapeutics for monkeypox has been significantly influenced by advances in virology, immunology, and biotechnology. The most widely used vaccines are modified vaccinia virus Ankara (MVA)-based vaccines, originally developed for smallpox but repurposed due to their cross-protective immunity against monkeypox. Next-generation vaccines with improved safety profiles and higher scalability are under development, aiming to provide long-term immunity with fewer side effects. In parallel, antiviral treatments such as tecovirimat (TPOXX), brincidofovir, and cidofovir have been identified as effective options for reducing symptom severity and preventing complications. The integration of monoclonal antibodies into treatment regimens is also being explored to enhance immune response in severe cases. Additionally, mRNA vaccine technology, which revolutionized COVID-19 vaccination, is being studied for its potential application in developing targeted monkeypox vaccines with rapid adaptability to emerging viral mutations. With increasing investment in research and development, the future of monkeypox vaccines and treatments looks promising, offering improved protection and treatment outcomes.How Are Government Policies and Public Health Strategies Influencing Market Growth?
Governments worldwide have implemented aggressive vaccination strategies and treatment access programs to control the spread of monkeypox, driving market demand for vaccines and therapeutics. Emergency use authorizations (EUAs) and fast-track approvals by regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have accelerated the availability of vaccines and antiviral drugs. National immunization programs have prioritized at-risk populations, leading to increased vaccine distribution and deployment of stockpiled smallpox vaccines. Public awareness campaigns and community health initiatives have further contributed to vaccination uptake and early treatment adoption. Additionally, funding initiatives by global health organizations, including the WHO and the Coalition for Epidemic Preparedness Innovations (CEPI), have supported large-scale clinical trials and expanded vaccine manufacturing capabilities. As governments continue to prioritize pandemic preparedness, investment in monkeypox vaccine and treatment research is expected to rise.What Are the Key Factors Driving the Growth of the Monkeypox Vaccine and Treatment Market?
The growth in the monkeypox vaccine and treatment market is driven by several factors, including the rising number of cases, advancements in vaccine development, and strong government intervention. The increased focus on pandemic preparedness and biosecurity measures has led to higher investments in vaccine research and global stockpiling of antiviral drugs. The expansion of production facilities and improvements in vaccine distribution channels are also fueling market growth. Additionally, the shift toward novel vaccine technologies, including mRNA-based formulations, is creating new opportunities for market expansion. The growing adoption of mass immunization programs and proactive testing strategies is further driving demand for monkeypox-related medical interventions. Furthermore, public-private partnerships between pharmaceutical companies and governments are ensuring rapid vaccine rollout and equitable access to treatments. As monkeypox continues to pose a public health threat, the market for vaccines and treatments is expected to witness sustained growth, driven by ongoing research and global vaccination initiatives.Report Scope
The report analyzes the Monkeypox Vaccine and Treatment market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (Monkeypox Vaccines, Monkeypox Drugs); Gender (Male Gender, Female Gender, Other Genders); Administration Route (Oral Administration, Injectable Administration); End-Use (Hospitals End-Use, Specialty Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Monkeypox Vaccines segment, which is expected to reach US$107.2 Million by 2030 with a CAGR of a 7.2%. The Monkeypox Drugs segment is also set to grow at 10.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $27.4 Million in 2024, and China, forecasted to grow at an impressive 12.5% CAGR to reach $34.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Monkeypox Vaccine and Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Monkeypox Vaccine and Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Monkeypox Vaccine and Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aegis Sciences Corporation, Applied DNA Clinical Labs, LLC, ARUP Laboratories, BayCare Laboratories, LLC, Baylor Scott & White Advanced Diagnostics Laboratory and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Monkeypox Vaccine and Treatment market report include:
- Abbott Laboratories
- AstraZeneca plc
- Bavarian Nordic A/S
- BioFactura, Inc.
- BioNTech SE
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- KM Biologics Co., Ltd.
- Merck & Co., Inc.
- Moderna, Inc.
- Novavax, Inc.
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- SIGA Technologies, Inc.
- Tonix Pharmaceuticals Holding Corp.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AstraZeneca plc
- Bavarian Nordic A/S
- BioFactura, Inc.
- BioNTech SE
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- KM Biologics Co., Ltd.
- Merck & Co., Inc.
- Moderna, Inc.
- Novavax, Inc.
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- SIGA Technologies, Inc.
- Tonix Pharmaceuticals Holding Corp.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 455 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
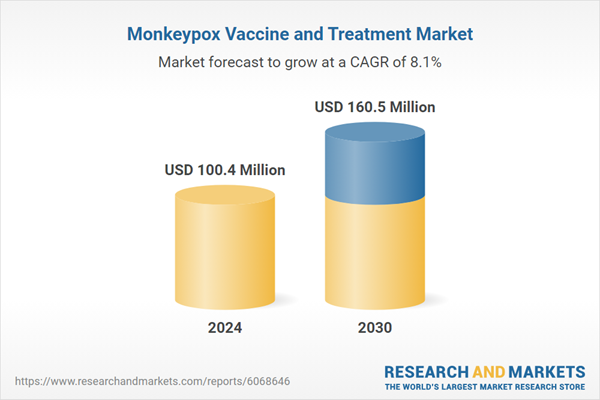
| Estimated Market Value ( USD | $ 100.4 Million |
| Forecasted Market Value ( USD | $ 160.5 Million |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |