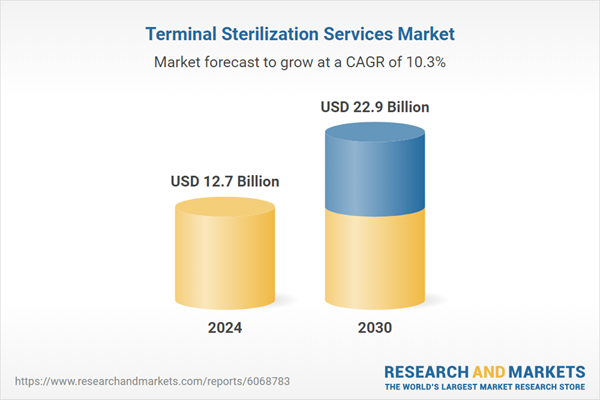
Global Terminal Sterilization Services Market - Key Trends & Drivers Summarized
Is Terminal Sterilization Becoming the Gold Standard in Medical Product Safety?
Terminal sterilization services have become an essential component of the global healthcare and medical device ecosystem, ensuring that products are sterile at the point of final packaging and distribution. Unlike aseptic processing, which attempts to maintain sterility throughout production, terminal sterilization occurs as the last step - offering a more definitive and validated method of microbial eradication. This process is critical for ensuring the safety of high-risk products such as surgical instruments, implants, syringes, catheters, and single-use medical disposables. Common sterilization techniques include ethylene oxide (EtO) sterilization, gamma irradiation, and electron beam (e-beam) sterilization, each selected based on material compatibility, penetration requirements, and regulatory standards. The demand for terminal sterilization services has surged amid rising global healthcare needs, stricter infection control protocols, and a growing emphasis on patient safety. In particular, the COVID-19 pandemic underscored the critical need for reliable sterilization of personal protective equipment (PPE) and diagnostic tools, catalyzing investments in both in-house and outsourced sterilization infrastructure. Moreover, regulatory agencies such as the U.S. FDA and EMA are tightening sterilization validation requirements, pushing manufacturers toward robust, externally validated sterilization pathways. With an ever-expanding range of complex and combination medical devices entering the market, terminal sterilization is fast becoming a non-negotiable standard in ensuring compliance and safeguarding public health.How Are Shifts in Medical Device Complexity Driving Demand for Specialized Sterilization?
The evolution of medical technology and device design has had a direct impact on the terminal sterilization services market. Modern medical devices are becoming increasingly intricate, involving complex geometries, sensitive materials, and integrated electronics that demand highly customized sterilization solutions. Traditional sterilization methods may degrade or alter certain polymers and bioactive coatings used in today's devices, prompting the development of low-temperature and alternative sterilization methods that preserve product integrity while achieving microbial efficacy. For instance, the rise of drug-device combination products - such as drug-eluting stents and prefilled syringes - requires sterilization processes that maintain the pharmacological properties of the embedded therapeutics. Furthermore, manufacturers must navigate challenges related to packaging materials, product shelf life, and moisture sensitivity, all of which influence the choice of sterilization modality. Contract sterilization providers are increasingly investing in R&D to create adaptable, scalable solutions tailored to specific device categories. Hospitals, pharmaceutical firms, and medical device manufacturers are also engaging in long-term partnerships with sterilization service providers to ensure consistency, traceability, and regulatory compliance throughout the product lifecycle. The growing volume of single-use and disposable products in surgical and diagnostic procedures further boosts demand for sterilization, especially in ambulatory surgical centers and emerging healthcare markets. As the boundaries of device innovation expand, so too does the need for precise, reliable, and validated terminal sterilization solutions that can keep pace with clinical complexity.Can Technology and Regulatory Pressures Reshape the Future of Sterilization Services?
Emerging technologies and evolving global regulations are reshaping the landscape of terminal sterilization services, compelling providers to innovate while maintaining compliance with rigorous safety standards. Ethylene oxide sterilization, long considered an industry staple, is under increasing scrutiny due to its environmental impact and potential carcinogenicity, prompting regulatory bodies to limit emissions and seek alternatives. In response, companies are investing in next-generation sterilization methods such as vaporized hydrogen peroxide (VHP), nitrogen dioxide (NO2), and low-temperature plasma sterilization, all of which offer reduced environmental footprints and enhanced safety profiles. Additionally, real-time monitoring, digital traceability, and process analytics are being integrated into sterilization workflows to provide end-to-end transparency and quality assurance. Cloud-based systems now allow remote monitoring of sterilization cycles, while AI-driven analytics are being piloted to predict maintenance needs and optimize energy consumption. The growing need for global harmonization of sterilization standards has prompted organizations like ISO and AAMI to update guidelines for validation, dose auditing, and process control - directly influencing how sterilization service providers structure their offerings. Investments in modular sterilization units and mobile sterilization services are also emerging to meet the demands of decentralized healthcare delivery. These shifts, while challenging, present opportunities for service providers to differentiate themselves through innovation, sustainability, and regulatory agility - ensuring that sterilization practices remain aligned with the future of healthcare delivery.What Factors Are Driving the Growth of the Terminal Sterilization Services Market?
The growth in the terminal sterilization services market is driven by several factors directly related to technological progress, evolving healthcare infrastructure, and shifting end-user requirements. A key driver is the rising global demand for sterile medical products, propelled by the increasing prevalence of chronic diseases, a growing elderly population, and the widespread use of minimally invasive surgical devices. The complexity and volume of single-use medical devices are expanding, especially in outpatient, home-care, and ambulatory settings - necessitating reliable sterilization to prevent cross-contamination. Outsourcing trends among pharmaceutical and medical device manufacturers are accelerating as companies seek specialized partners for high-volume, validated sterilization services that meet global compliance standards. Technological advancements in sterilization equipment - such as automated chambers, environmentally friendly sterilants, and digitized process validation - are enhancing efficiency and reducing operational costs. Regulatory mandates regarding sterility assurance levels (SAL), emission control, and traceability are further compelling healthcare product companies to adopt advanced terminal sterilization protocols. Additionally, the increasing stringency of audits and product recalls related to contamination concerns is reinforcing the market need for comprehensive, risk-managed sterilization services. Growth in biotechnology, tissue engineering, and regenerative medicine sectors is also expanding the need for customized sterilization methods that accommodate biological sensitivity. Geographically, rising healthcare investments in Asia-Pacific, Latin America, and the Middle East are driving demand for third-party sterilization providers with the infrastructure and expertise to serve emerging markets. These converging trends are fueling a sustained expansion of the terminal sterilization services market across both established and developing healthcare economies.Report Scope
The report analyzes the Terminal Sterilization Services market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Ethylene Oxide, Irradiation, Moist Heat Terminal Sterilization, Others); End-Use (Hospitals & Clinics, Pharma & Nutraceuticals, Medical Device Manufacturing / Packaging, Academic Research Institutes, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Ethylene Oxide segment, which is expected to reach US$10.9 Billion by 2030 with a CAGR of a 12.6%. The Irradiation segment is also set to grow at 8.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.5 Billion in 2024, and China, forecasted to grow at an impressive 14.4% CAGR to reach $4.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Terminal Sterilization Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Terminal Sterilization Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Terminal Sterilization Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Advanced Micro Devices, Inc. (AMD), Alibaba Group Holding Limited, Analog Devices, Inc., Apple Inc., ARM Holdings plc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Terminal Sterilization Services market report include:
- 3M Company
- Advanced Sterilization Products Services, Inc. (Fortive)
- Andersen Sterilizers
- B. Braun Medical Inc.
- Belimed, Inc. (Metall Zug Group)
- Cantel Medical (Subsidiary of STERIS)
- Cretex Medical
- E-BEAM Services, Inc.
- Fedegari Autoclavi S.p.A.
- Getinge AB
- Johnson & Johnson
- Lonza Group
- Matachana Group
- Medistri SA
- Nelson Laboratories, LLC (A Sotera Health Company)
- Noxilizer, Inc.
- Olympus Corporation
- Prince Sterilization Services, LLC
- Pro-Tech Design & Manufacturing, Inc.
- Providence Enterprise
- SteriTek
- STERIS plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Company
- Advanced Sterilization Products Services, Inc. (Fortive)
- Andersen Sterilizers
- B. Braun Medical Inc.
- Belimed, Inc. (Metall Zug Group)
- Cantel Medical (Subsidiary of STERIS)
- Cretex Medical
- E-BEAM Services, Inc.
- Fedegari Autoclavi S.p.A.
- Getinge AB
- Johnson & Johnson
- Lonza Group
- Matachana Group
- Medistri SA
- Nelson Laboratories, LLC (A Sotera Health Company)
- Noxilizer, Inc.
- Olympus Corporation
- Prince Sterilization Services, LLC
- Pro-Tech Design & Manufacturing, Inc.
- Providence Enterprise
- SteriTek
- STERIS plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 278 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 12.7 Billion |
| Forecasted Market Value ( USD | $ 22.9 Billion |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Global |