Global 'Cancer Biological Therapy' Market - Key Trends & Drivers Summarized
How Is Biological Therapy Transforming The Cancer Treatment Paradigm?
Cancer biological therapy represents a pivotal shift from traditional chemotherapies to more targeted and immune-based approaches, leveraging the body's natural mechanisms or engineered biological agents to combat cancer cells. These therapies include monoclonal antibodies, cytokine therapies, cancer vaccines, oncolytic viruses, and CAR-T cell therapies. Unlike cytotoxic drugs, biological therapies offer precision targeting, potentially reducing collateral damage to healthy tissues and improving patient quality of life. With rising global cancer incidence, there's a pressing need for treatments that go beyond broad-spectrum options - particularly for refractory and metastatic cancers. Biologics are proving effective across a range of malignancies including lymphomas, melanoma, breast cancer, and lung cancer. The integration of diagnostics and biomarkers into treatment decisions has enabled biological therapies to be tailored to genetic profiles, increasing their success rates. Combination therapies involving checkpoint inhibitors and monoclonals are also opening new frontiers. Moreover, immunotherapy - an important subset of biological therapy - is rapidly becoming a first-line option in many countries, signaling a fundamental evolution in oncology treatment strategy.What Scientific Innovations Are Expanding The Therapeutic Reach Of Biologics?
The cancer biological therapy landscape is being redefined by breakthroughs in cell engineering, genetic editing, and antibody development. The advent of bispecific antibodies, which can simultaneously bind to cancer cells and T-cells, is creating potent new treatment modalities. CAR-T cell therapy, initially used in hematologic malignancies, is being adapted for solid tumors through improved tumor-targeting and persistence technologies. Personalized neoantigen vaccines are being developed to boost immune recognition of tumor-specific mutations. Synthetic biology is enabling programmable immune cells with built-in safety switches and dual-function mechanisms. CRISPR gene editing is being used to enhance the performance of adoptive cell therapies, minimizing immune escape by tumors. Additionally, advances in protein engineering are yielding antibody-drug conjugates (ADCs) that combine high specificity with powerful cytotoxic payloads. AI-driven predictive modeling is facilitating the design of biologics with optimized pharmacokinetics and minimal toxicity. These innovations are not just expanding the types of cancers that can be treated but are also enabling therapy development that is faster, more cost-effective, and more targeted than ever before.How Are Regulatory Shifts And Patient Demands Reshaping Cancer Biologics?
The regulatory landscape for cancer biological therapies is undergoing transformation to keep pace with innovation, leading to faster approval pathways like FDA’ s Breakthrough Therapy and EMA’ s PRIME designations. Accelerated approvals for drugs such as checkpoint inhibitors and CAR-T therapies underscore the urgency and impact of these treatments. However, regulators are also enforcing stringent post-market surveillance and real-world evidence requirements due to the complexity and novelty of biologics. Patient advocacy has become a force in shaping treatment access, with increasing pressure on healthcare systems to subsidize or reimburse cutting-edge therapies. The rise of value-based healthcare is prompting payers to assess outcomes-based models for biologics reimbursement. In parallel, there's increasing emphasis on diversity in clinical trials, particularly for immunotherapies, which can behave differently across populations. Ethical considerations around gene-modified therapies are also leading to stricter oversight in manufacturing and consent protocols. Furthermore, the growing role of companion diagnostics in therapy selection is pushing biologics companies to partner closely with diagnostic firms to co-develop biomarker-led strategies. This holistic regulatory-patient dynamic is accelerating innovation while demanding higher transparency and safety.The Growth In The Cancer Biological Therapy Market Is Driven By Several Factors…
… including rising cancer prevalence, increased adoption of personalized medicine, and growing investment in immuno-oncology pipelines. Demand is surging for biologics in difficult-to-treat cancers, particularly those resistant to standard chemotherapy or radiation. The success of PD-1/PD-L1 inhibitors and CAR-T cell products has spurred a wave of biotech and pharma investment in next-gen immunotherapies. Pharmaceutical giants are expanding their biological portfolios through acquisitions, while governments are funding cancer research through public-private partnerships. In developing regions, the rollout of healthcare infrastructure is increasing access to advanced therapies, supported by rising awareness and early diagnosis initiatives. Trends in consumer behavior show patients increasingly seeking therapies with fewer side effects and longer remission durations. The expansion of clinical trials globally, especially in Asia-Pacific, is facilitating the entry of biologics into new cancer subtypes and indications. Tele-oncology and digital health platforms are enhancing patient management and follow-up, supporting biologic adherence and outcomes tracking. Together, these drivers are forging a robust foundation for the continued evolution of the cancer biological therapy market.Report Scope
The report analyzes the Cancer Biological Therapy market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Monoclonal Antibodies, Cancer Growth Blockers, Blood Cell Growth Factors, Cytokines, Vaccines); Administration Route (Oral Administration, Injectable Administration).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Monoclonal Antibodies segment, which is expected to reach US$69.9 Billion by 2030 with a CAGR of a 6.9%. The Cancer Growth Blockers segment is also set to grow at 5.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $33.2 Billion in 2024, and China, forecasted to grow at an impressive 9.6% CAGR to reach $35.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cancer Biological Therapy Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cancer Biological Therapy Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cancer Biological Therapy Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amcor PLC, Ardagh Group SA, Ardagh Metal Packaging (AMP), Ball Corporation, Canpack S.A. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Cancer Biological Therapy market report include:
- AbbVie, Inc.
- Amgen, Inc.
- Astellas Pharma, Inc.
- AstraZeneca Plc
- Biogen, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- GSK Plc
- Incyte Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie, Inc.
- Amgen, Inc.
- Astellas Pharma, Inc.
- AstraZeneca Plc
- Biogen, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- GSK Plc
- Incyte Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 211 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
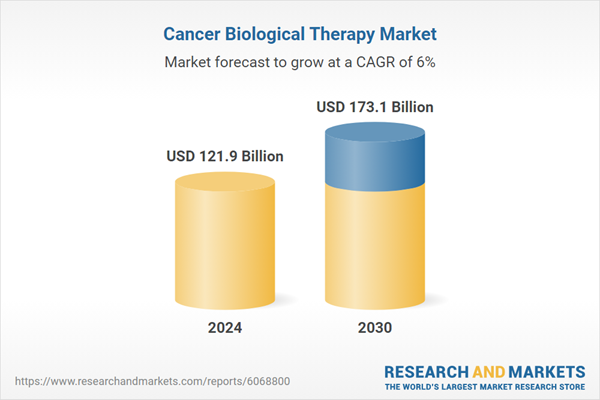
| Estimated Market Value ( USD | $ 121.9 Billion |
| Forecasted Market Value ( USD | $ 173.1 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |