Global Clinical Trials Support Services Market - Key Trends & Drivers Summarized
How Are Support Services Enhancing Clinical Trial Efficiency?
Clinical trials support services encompass a broad range of operational, regulatory, and logistical solutions that ensure the successful execution of research studies. These services include data management, regulatory compliance, medical writing, and patient support programs. With the increasing complexity of clinical trials, sponsors are increasingly relying on specialized support service providers to optimize study timelines, reduce costs, and maintain regulatory adherence. The shift toward decentralized and hybrid trials is also expanding the need for virtual support services, such as remote monitoring, eConsent platforms, and digital patient engagement solutions.What Innovations Are Transforming Clinical Trials Support Services?
The adoption of AI-driven data analytics, blockchain-powered regulatory tracking, and cloud-based document management platforms is significantly improving the efficiency of trial support services. Automated data verification tools are reducing errors in clinical documentation, ensuring compliance with Good Clinical Practice (GCP) guidelines. AI-powered protocol optimization software is enabling real-time adjustments to trial designs, improving study feasibility and patient retention. Additionally, virtual patient support services, such as telehealth consultations and mobile health apps, are enhancing participant engagement and adherence.What’ s Driving the Growth of the Clinical Trials Support Services Market?
The growth in the clinical trials support services market is driven by increasing regulatory requirements, the rise of digital health technologies, and the expansion of global clinical research initiatives. The demand for real-time data analysis and protocol optimization is fueling investments in AI-powered support platforms. The shift toward decentralized trials is expanding the need for remote site monitoring and virtual patient engagement solutions. Additionally, regulatory agencies are emphasizing the importance of transparency in clinical trials, encouraging sponsors to invest in compliance-driven support services. As clinical research continues to evolve, the demand for integrated, technology-driven support solutions is expected to rise, propelling market growth.Report Scope
The report analyzes the Clinical Trials Support Services market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase Type (Phase I, Phase II, Phase III, Phase IV); Service Type (Clinical Trial Site Management Services, Patient Recruitment Management Services, Data Management Services, Administrative Staff Services, IRB Services, Other Services); End-Use (Pharmaceutical and Biopharmaceutical Companies End-Use, Medical Device Companies End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$16.7 Billion by 2030 with a CAGR of a 7.6%. The Phase II Clinical Trials segment is also set to grow at 6.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6.3 Billion in 2024, and China, forecasted to grow at an impressive 10.6% CAGR to reach $7.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Clinical Trials Support Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Clinical Trials Support Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Clinical Trials Support Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Adaptive Clinical Systems, Antidote Technologies, AutoCruitment, BBK Worldwide, Clariness and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Clinical Trials Support Services market report include:
- Celerion
- Charles River Laboratories International Inc.
- Covance Inc.
- ICON plc
- IQVIA
- KCR
- Labcorp Drug Development
- Linical Co., Ltd.
- Medidata Solutions
- Medpace Holdings Inc.
- Novotech
- Parexel International Corporation
- Pharm-Olam International
- PPD (Pharmaceutical Product Development)
- PRA Health Sciences
- PSI CRO
- Syneos Health
- Veeva Systems
- Worldwide Clinical Trials
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Celerion
- Charles River Laboratories International Inc.
- Covance Inc.
- ICON plc
- IQVIA
- KCR
- Labcorp Drug Development
- Linical Co., Ltd.
- Medidata Solutions
- Medpace Holdings Inc.
- Novotech
- Parexel International Corporation
- Pharm-Olam International
- PPD (Pharmaceutical Product Development)
- PRA Health Sciences
- PSI CRO
- Syneos Health
- Veeva Systems
- Worldwide Clinical Trials
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 379 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
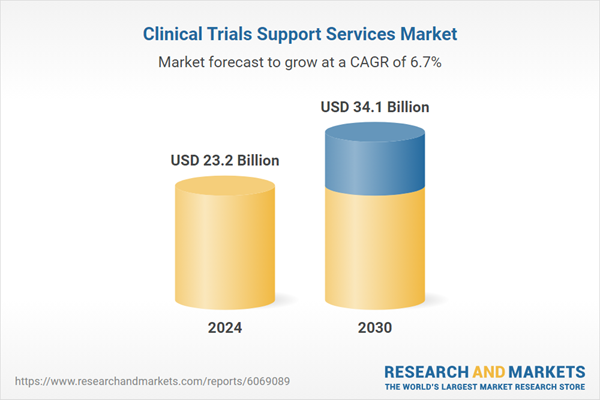
| Estimated Market Value ( USD | $ 23.2 Billion |
| Forecasted Market Value ( USD | $ 34.1 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |