Global Mental Health Clinical Trials Market - Key Trends & Drivers Summarized
What Are Mental Health Clinical Trials and Why Are They Important?
Mental health clinical trials are research studies conducted to evaluate the safety, efficacy, and overall impact of new treatments or interventions for mental health conditions, such as depression, anxiety, schizophrenia, and bipolar disorder. These trials are a critical component of the drug development process, as they help determine whether a proposed treatment can effectively alleviate symptoms, improve quality of life, and potentially offer new solutions for individuals who have not responded to existing therapies. Clinical trials are typically conducted in several phases, ranging from small-scale Phase I trials focusing on safety, to large-scale Phase III trials aimed at confirming the efficacy of the treatment.Mental health clinical trials often involve different types of interventions, such as pharmaceuticals, behavioral therapies, medical devices, or digital health solutions. The research is conducted under stringent ethical guidelines, with an emphasis on protecting the rights and well-being of participants. As mental health issues continue to rise globally, the importance of these trials has never been more apparent. They provide the data necessary to inform treatment guidelines, help healthcare professionals make more accurate diagnoses, and ultimately improve outcomes for individuals suffering from mental health disorders.
What Are the Latest Trends in Mental Health Clinical Trials?
One of the most significant trends in mental health clinical trials is the increasing use of digital health technologies. With the rise of telemedicine, wearable devices, and mobile health apps, many mental health trials are incorporating digital tools to collect real-time data on patient behavior, treatment adherence, and overall mental well-being. These technologies allow for more frequent and detailed monitoring, enabling researchers to track symptoms, side effects, and recovery progress in ways that were not possible in traditional clinical settings. Furthermore, digital health tools provide the flexibility to conduct remote trials, reducing barriers to participation, particularly for individuals in underserved or rural areas.Additionally, the growing interest in personalized medicine is transforming mental health clinical trials. Researchers are increasingly focusing on tailoring treatments to an individual's genetic makeup, lifestyle, and specific mental health conditions. This approach, often referred to as precision psychiatry, aims to develop more effective therapies that can provide better outcomes for patients based on their unique characteristics. For instance, genetic testing is being integrated into clinical trials to identify biomarkers that predict which patients are more likely to respond to specific medications or therapies. This shift towards personalized care promises to increase the success rates of clinical trials and ultimately lead to more targeted, effective treatments for mental health disorders.
Why Is the Demand for Mental Health Clinical Trials Increasing?
The demand for mental health clinical trials has been steadily increasing due to the rising global burden of mental health disorders. According to the World Health Organization, depression is now the leading cause of disability worldwide, and anxiety disorders are also among the most common mental health conditions. The COVID-19 pandemic has exacerbated these issues, with significant spikes in mental health conditions due to isolation, stress, and uncertainty. This has created an urgent need for new treatment options and has catalyzed investment in mental health research. As a result, pharmaceutical companies, research institutions, and healthcare providers are ramping up efforts to explore novel therapies, both pharmaceutical and non-pharmaceutical, to address the growing demand.Additionally, there is an increased recognition of mental health as a critical component of overall well-being, which has further amplified the need for clinical trials. Governments, organizations, and advocacy groups are putting more emphasis on mental health, urging policymakers to prioritize funding for mental health research. This broader societal focus on mental health has led to greater collaboration across sectors, fostering innovation and encouraging the development of more treatment options. Furthermore, there is growing patient advocacy for better treatments, as many individuals with mental health conditions feel that current therapies do not provide sufficient relief. These factors have created a strong demand for more robust and varied clinical trials aimed at addressing the diverse needs of mental health patients.
What Are the Key Growth Drivers in the Mental Health Clinical Trials Market?
The growth in the mental health clinical trials market is driven by several factors, primarily the increasing prevalence of mental health disorders worldwide. As the global burden of conditions such as depression, anxiety, bipolar disorder, and schizophrenia continues to rise, there is an urgent need for new treatments and interventions. This has led to higher levels of investment from both public and private sectors in mental health research, supporting a more diverse range of clinical trials. The rising demand for more effective therapies, coupled with the high unmet medical need in mental health, continues to propel growth in the market.Another significant driver is the expansion of digital and decentralized clinical trials. The integration of digital health technologies, such as telemedicine, remote monitoring tools, and mobile applications, allows for more flexible and scalable clinical trials. These technologies facilitate greater patient recruitment, improve data collection, and enable trials to be conducted across broader geographic regions. The use of these tools not only enhances the efficiency of trials but also opens up access to patients who may not have been able to participate in traditional trials due to logistical barriers or geographic limitations.
Additionally, regulatory advancements have played a pivotal role in accelerating the growth of the mental health clinical trials market. Regulatory bodies, such as the FDA and EMA, have recognized the urgency of addressing mental health disorders and have streamlined approval processes for novel treatments, including digital therapies and other non-traditional interventions. These regulatory improvements have encouraged more companies to enter the mental health space, bringing innovative solutions to market faster. Furthermore, the growing trend of personalized medicine, driven by genetic and biomarker research, is helping to create more targeted trials that improve the precision of treatments and reduce trial failure rates. As the mental health clinical trials landscape continues to evolve, these factors are expected to fuel further growth and innovation in the market.
Report Scope
The report analyzes the Mental Health Clinical Trials market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Phase (Phase I, Phase II, Phase III, Phase IV); Disorder (Anxiety Disorders, Depression, Bipolar affective disorder, Dissociation & dissociative disorders, Schizophrenia, Others); Study Design (Interventional, Observational, Others); Sponsor (Pharma & Biotech Companies, Government Agencies, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$1.9 Billion by 2030 with a CAGR of a 5.3%. The Phase II Clinical Trials segment is also set to grow at 8.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $846.3 Million in 2024, and China, forecasted to grow at an impressive 10.4% CAGR to reach $943.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Mental Health Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Mental Health Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Mental Health Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Caidya, Cenexel Clinical Research, Inc., COMPASS Pathways, Corcept Therapeutics, Inc., Eli Lilly and Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Mental Health Clinical Trials market report include:
- Caidya
- Cenexel Clinical Research, Inc.
- COMPASS Pathways
- Corcept Therapeutics, Inc.
- Eli Lilly and Company
- Fortrea, Inc.
- ICON Plc
- IQVIA, Inc.
- Kyocera Corporation
- Labcorp Drug Development
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Caidya
- Cenexel Clinical Research, Inc.
- COMPASS Pathways
- Corcept Therapeutics, Inc.
- Eli Lilly and Company
- Fortrea, Inc.
- ICON Plc
- IQVIA, Inc.
- Kyocera Corporation
- Labcorp Drug Development
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 482 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
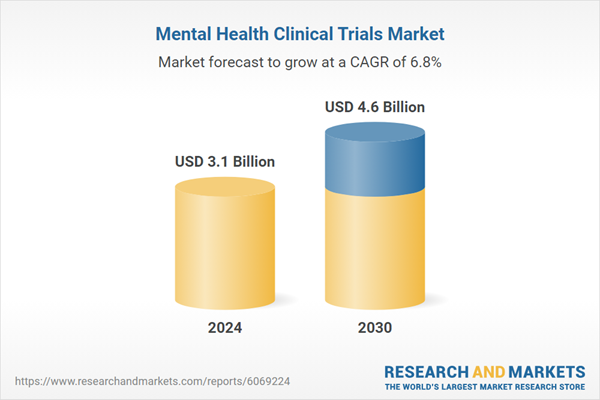
| Estimated Market Value ( USD | $ 3.1 Billion |
| Forecasted Market Value ( USD | $ 4.6 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |