Global Dermal Toxicity Testing Market - Key Trends & Drivers Summarized
Why Is The Demand For Dermal Toxicity Testing Increasing Rapidly?
The global dermal toxicity testing market is experiencing rapid growth due to the rising need for safe and effective skincare, pharmaceuticals, and chemical products. As regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Chemicals Agency (ECHA) impose stringent safety standards, companies must ensure that their products do not cause skin irritation, sensitization, or long-term dermal damage. The cosmetics and personal care industry, in particular, has witnessed a surge in demand for cruelty-free and non-animal-tested products, further driving the adoption of alternative dermal toxicity testing methods. Additionally, increasing consumer awareness of the potential adverse effects of synthetic chemicals has encouraged manufacturers to prioritize rigorous safety evaluations. With the expanding landscape of dermatological research and the rise in skin-related disorders, the demand for comprehensive dermal toxicity testing continues to grow.How Are Technological Innovations Transforming The Dermal Toxicity Testing Market?
Technological advancements have significantly revolutionized the dermal toxicity testing landscape, introducing more reliable, ethical, and cost-effective solutions. The adoption of in vitro testing methods, such as 3D human skin models, has provided a superior alternative to traditional animal testing, offering higher accuracy in predicting human skin reactions. Additionally, high-throughput screening (HTS) techniques and advanced bioinformatics tools have accelerated toxicity analysis, allowing researchers to assess multiple compounds simultaneously. The integration of artificial intelligence (AI) and machine learning in toxicity prediction has further enhanced data analysis, improving efficiency and precision in toxicity assessment. Furthermore, regulatory bodies are increasingly advocating for in silico (computer-based) modeling techniques, which enable virtual simulations of skin interactions with chemicals. These technological breakthroughs are shaping the future of dermal toxicity testing, ensuring safer and more ethical product development.What Are The Major Challenges Affecting The Dermal Toxicity Testing Market?
Despite the progress in the field, several challenges continue to affect the growth and standardization of dermal toxicity testing. One of the primary obstacles is the complexity of human skin biology, making it difficult to create universally applicable in vitro models. While alternative methods have made strides in replacing animal testing, regulatory validation and global acceptance of these models remain slow, delaying widespread adoption. Additionally, the high costs associated with advanced testing techniques and the need for skilled professionals pose financial and operational barriers for smaller companies. Moreover, discrepancies in safety regulations across different countries create compliance challenges for multinational businesses, necessitating continuous adaptation to region-specific testing requirements. Addressing these issues through increased regulatory harmonization, investment in research, and cost-effective innovations will be essential for the sustained expansion of the dermal toxicity testing market.What Factors Are Driving The Growth Of The Dermal Toxicity Testing Market?
The growth in the dermal toxicity testing market is driven by several factors, including rising regulatory scrutiny, increasing consumer demand for safe and ethical products, and advancements in alternative testing methodologies. The growing adoption of in vitro and in silico testing approaches, along with AI-driven predictive modeling, has enhanced the reliability and efficiency of toxicity assessments. Additionally, the expansion of the pharmaceutical, cosmetic, and chemical industries has intensified the need for stringent safety evaluations, propelling market growth. The push for cruelty-free testing, backed by government regulations and advocacy groups, has accelerated the transition away from animal testing methods. Furthermore, increased research funding and collaborations between regulatory agencies and private organizations are fostering the development of innovative dermal toxicity testing solutions. With continuous technological progress and a rising focus on safety, the market is poised for sustained growth in the coming years.Report Scope
The report analyzes the Dermal Toxicity Testing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Dermal Toxicity Testing Services, Dermal Toxicity Testing Products); Testing Method (In-Vitro Testing Method, In-Vivo Testing Method, In-Silico Modeling Testing Method); Test Type (Skin Sensitization Tests, Skin Irritation Tests, Skin Corrosion Tests, Phototoxicity Tests); End-Use (Healthcare industry End-Use, Cosmetics and Personal Care Products End-Use, Chemical End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Dermal Toxicity Testing Services segment, which is expected to reach US$2.4 Billion by 2030 with a CAGR of a 8.9%. The Dermal Toxicity Testing Products segment is also set to grow at 5.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $699.1 Million in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $654.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Dermal Toxicity Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Dermal Toxicity Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Dermal Toxicity Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Airjoi, Bamboo Charcoal Bags, Bamboolik, Basic Concepts, Breathe Green and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Dermal Toxicity Testing market report include:
- Abbott Laboratories
- Agilent Technologies Inc.
- Bio-Rad Laboratories Inc.
- Catalent, Inc.
- Charles River Laboratories International, Inc.
- Covance Inc. (now part of Labcorp)
- Cyprotex (a subsidiary of Evotec SE)
- Eurofins Scientific Inc.
- Evotec SE
- GE Healthcare
- Intertek Group plc
- InVivo Biosystems
- MatTek Corporation
- Merck KGaA
- Promega Corporation
- Qiagen N.V.
- QIMA Ltd.
- Quest Diagnostics Incorporated
- SGS S.A.
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Agilent Technologies Inc.
- Bio-Rad Laboratories Inc.
- Catalent, Inc.
- Charles River Laboratories International, Inc.
- Covance Inc. (now part of Labcorp)
- Cyprotex (a subsidiary of Evotec SE)
- Eurofins Scientific Inc.
- Evotec SE
- GE Healthcare
- Intertek Group plc
- InVivo Biosystems
- MatTek Corporation
- Merck KGaA
- Promega Corporation
- Qiagen N.V.
- QIMA Ltd.
- Quest Diagnostics Incorporated
- SGS S.A.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
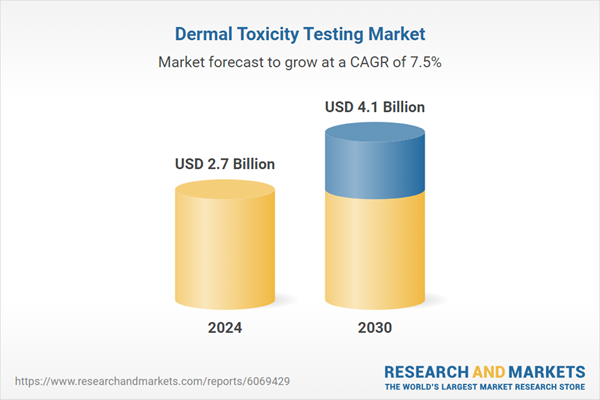
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 4.1 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |