Global Endoscopy Operative Devices Market - Key Trends & Drivers Summarized
Why Are Endoscopy Operative Devices Becoming Central to Modern Surgical Strategies?
The global endoscopy operative devices market is undergoing a substantial evolution, fueled by a growing shift toward minimally invasive surgeries (MIS) and the increasing sophistication of endoscopic techniques across multiple clinical specialties. These devices - encompassing biopsy forceps, graspers, scissors, snares, electrosurgical instruments, staplers, and suturing systems - serve as the primary tools through which diagnostic and therapeutic interventions are performed within the endoscopic field. As procedural complexity and clinical expectations rise, the demand for high-precision, ergonomically advanced, and multi-functional operative devices is accelerating at pace. Hospitals, outpatient centers, and specialty clinics are prioritizing investments in instrument portfolios that can enhance surgical efficiency, reduce procedure time, and enable greater procedural versatility.From a commercial standpoint, the market is seeing a marked transition from reusable to single-use and semi-disposable devices. This shift is driven by a heightened emphasis on infection control, cost predictability, and workflow standardization. In parallel, robotic-assisted endoscopic surgery is gaining prominence, creating new demand for compatible operative tools that can integrate with digital consoles and support instrument articulation within confined anatomical spaces. The convergence of these trends is transforming endoscopy operative devices from simple mechanical tools into integral components of digitally augmented, high-performance surgical ecosystems.
How Are Use-Case Specialization and Clinical Demand Shaping Product Evolution?
The endoscopy operative devices market is increasingly being driven by the procedural diversification occurring across clinical fields. Gastroenterology, urology, gynecology, pulmonology, ENT, and bariatric surgery each have distinct anatomical and functional requirements - prompting OEMs to engineer instruments that meet highly specific end-use conditions. For example, advanced energy devices for polypectomy, precision snares for EMR/ESD (endoscopic mucosal resection/submucosal dissection), and flexible staplers for bariatric endoscopy are seeing rapid uptake. Additionally, devices with integrated irrigation and suction, insulated tips, and multifunctional capabilities are improving workflow efficiency by minimizing the need for multiple instrument exchanges.As healthcare providers aim to expand procedural capabilities while maintaining cost discipline, there's a growing preference for modular systems and multi-instrument kits. These product configurations offer flexibility across various procedures without inflating procurement or sterilization costs. Moreover, endoscopists and minimally invasive surgeons are increasingly influencing purchasing decisions, placing greater emphasis on factors such as tactile feedback, ergonomic design, and device maneuverability - all of which are pushing manufacturers to rethink traditional device engineering. This clinical and ergonomic convergence is fueling next-generation product development with enhanced user control, articulation, and adaptive tissue response technologies.
How Is Innovation Transforming the Competitive Landscape and Enabling Differentiation?
Innovation is now the cornerstone of competitive strategy in the endoscopy operative devices market. Market leaders and challengers alike are leveraging advanced materials, robotics-compatible designs, and digital enhancements to build premium product lines. High-performance coatings that improve tissue glide, anti-fog tips, radiofrequency-enabled surgical tools, and devices embedded with sensors for real-time feedback are becoming increasingly mainstream. These innovations not only improve clinical outcomes but also enhance the user experience, a key consideration for institutions seeking to attract and retain skilled surgical talent.Another critical differentiator is platform compatibility. Devices that can seamlessly integrate with flexible and rigid endoscopes, as well as robotic systems, are seeing heightened adoption in high-tech ORs. Furthermore, vendors are developing solutions that support data capture, instrument tracking, and usage analytics - all aligned with broader trends in surgical digitization. Companies are also expanding their aftermarket service models, offering bundled maintenance, training, and sterilization services to enhance customer loyalty and create high-retention sales cycles. In this fast-evolving competitive environment, the ability to deliver end-to-end procedural value, rather than just single-use instrumentation, is emerging as a key success factor.
What Are the Key Drivers Accelerating Market Growth Across Regions and Specialties?
The growth in the endoscopy operative devices market is driven by several factors directly related to technology advancement, procedural expansion, and specialty-specific needs. First, the global increase in diagnostic and therapeutic endoscopic procedures - particularly in colorectal screening, gastroesophageal reflux disease (GERD) treatment, bariatric endoscopy, and oncological interventions - is dramatically boosting demand for high-precision operative instruments. As health systems prioritize minimally invasive techniques for their cost-efficiency and quicker recovery times, the role of advanced operative devices in supporting clinical decision-making and execution is becoming more prominent.Technology is another major accelerator. Devices with enhanced ergonomics, multi-functionality, and robotic compatibility are unlocking new clinical possibilities and setting new standards for surgical outcomes. As hospitals transition toward integrated OR environments, instrument interoperability and digital compatibility are no longer optional but essential. Additionally, the rise of outpatient and ambulatory care centers has created strong demand for cost-effective, sterile, and easy-to-use devices that support high procedure volumes with minimal reprocessing requirements.
Geographically, emerging economies are experiencing strong uptake of endoscopy operative devices due to expanding endoscopy programs, infrastructure modernization, and greater awareness of MIS benefits. Governments and private hospital networks across Asia-Pacific, Latin America, and parts of Africa are investing in specialized surgical capabilities, often supported by global partnerships and training programs. At the same time, developed markets are increasingly focused on device innovation, workflow integration, and clinical outcomes - driving premiumization and platform-based procurement. Together, these dynamics position the endoscopy operative devices market for robust, sustained global growth across both high-end and cost-sensitive healthcare environments.
Report Scope
The report analyzes the Endoscopy Operative Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Reusable Operative Devices, Disposable Operative Devices).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Reusable Operative Devices segment, which is expected to reach US$17.3 Billion by 2030 with a CAGR of a 3.9%. The Disposable Operative Devices segment is also set to grow at 3.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $5.8 Billion in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $5.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Endoscopy Operative Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Endoscopy Operative Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Endoscopy Operative Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Arthrex, Inc., ConMed Corporation, Hologic, Inc., KARL STORZ SE & Co. KG, Medtronic Plc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Endoscopy Operative Devices market report include:
- Ambu A/S
- Applied Medical
- Arthrex, Inc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Cantel Medical Corp.
- CapsoVision, Inc.
- Coloplast A/S
- CONMED Corporation
- Cook Medical
- EndoChoice (Boston Scientific)
- EndoMaster Pte Ltd
- ERBE Elektromedizin GmbH
- Ethicon (Johnson & Johnson)
- Fujifilm Holdings Corporation
- Hologic, Inc.
- HOYA Corporation
- Intuitive Surgical, Inc.
- Johnson & Johnson
- KARL STORZ SE & Co. KG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ambu A/S
- Applied Medical
- Arthrex, Inc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Cantel Medical Corp.
- CapsoVision, Inc.
- Coloplast A/S
- CONMED Corporation
- Cook Medical
- EndoChoice (Boston Scientific)
- EndoMaster Pte Ltd
- ERBE Elektromedizin GmbH
- Ethicon (Johnson & Johnson)
- Fujifilm Holdings Corporation
- Hologic, Inc.
- HOYA Corporation
- Intuitive Surgical, Inc.
- Johnson & Johnson
- KARL STORZ SE & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 172 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
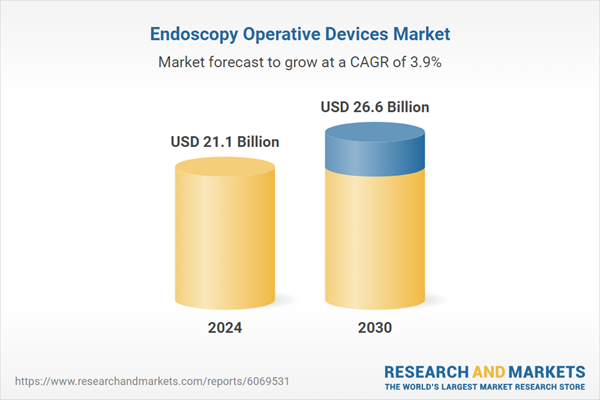
| Estimated Market Value ( USD | $ 21.1 Billion |
| Forecasted Market Value ( USD | $ 26.6 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |