Global Non-Alcoholic Steatohepatitis (NASH) Clinical Trials Market - Key Trends & Drivers Summarized
Why Is the Demand for NASH Clinical Trials Growing Rapidly?
The rising global burden of non-alcoholic steatohepatitis (NASH) has fueled the demand for clinical trials aimed at developing effective therapeutic interventions for this complex liver disease. Unlike other metabolic disorders, NASH currently has no FDA-approved treatments, making clinical research essential for identifying potential drug candidates that can prevent liver fibrosis and disease progression. Pharmaceutical and biotechnology companies are investing heavily in NASH drug development, with multiple compounds in Phase II and III clinical trials targeting different disease pathways, including inflammation, fibrosis, and lipid metabolism. The need for diverse patient populations in trials is also increasing, as researchers seek to understand how genetic, lifestyle, and metabolic factors influence disease progression and treatment response. Additionally, regulatory agencies are providing fast-track designations and priority reviews for promising NASH therapies, encouraging investment in large-scale clinical trials. As the healthcare industry prioritizes metabolic liver disease research, the NASH clinical trials market is experiencing significant expansion, paving the way for the next generation of liver therapeutics.What Challenges Are Impacting the Progress of NASH Clinical Trials?
Despite increased investment in NASH research, clinical trials face several hurdles that affect drug development timelines and success rates. One of the most significant challenges is patient recruitment and retention, as NASH is often asymptomatic in its early stages, leading to difficulties in identifying eligible trial participants. The reliance on liver biopsy as a primary endpoint for assessing treatment efficacy also poses a challenge, as it limits patient willingness to participate in invasive procedures. Additionally, the heterogeneity of NASH pathophysiology complicates drug development, as different mechanisms contribute to disease progression in various patient subgroups. Regulatory uncertainties and varying approval criteria across global markets further slow down trial progression, requiring companies to navigate complex compliance frameworks. Moreover, the high failure rate of NASH drug candidates in late-stage trials has raised concerns about the commercial viability of potential treatments. Addressing these challenges will require improved trial designs, better non-invasive biomarkers, and greater collaboration between stakeholders to accelerate drug discovery efforts.How Are Innovations and Emerging Therapies Shaping the Future of NASH Clinical Trials?
Advancements in drug development and clinical trial methodologies are transforming NASH research, increasing the likelihood of successful therapeutic breakthroughs. Precision medicine approaches are enabling patient stratification based on genetic and metabolic profiles, allowing researchers to tailor treatments to specific disease subtypes. The use of AI-powered clinical trial management systems is improving patient recruitment, reducing dropout rates, and optimizing trial efficiency. Non-invasive imaging techniques, such as multiparametric MRI and transient elastography, are being integrated into trials to assess liver health without requiring biopsies, improving patient compliance. Additionally, combination therapies targeting multiple disease pathways simultaneously are showing promise in addressing the complexity of NASH progression. The emergence of novel drug classes, including FXR agonists, PPAR agonists, and anti-fibrotic agents, is further diversifying the therapeutic landscape, increasing the chances of successful treatment approvals. As these innovations continue to evolve, they are reshaping the NASH clinical trials market, accelerating the development of effective and accessible liver disease treatments.What Is Driving the Growth of the NASH Clinical Trials Market?
The growth in the NASH clinical trials market is driven by several factors, including the urgent need for FDA-approved treatments, increasing prevalence of metabolic liver diseases, and advancements in non-invasive diagnostic tools. The expansion of pharmaceutical investments in metabolic disorder therapeutics is fueling trial activity, with multiple biopharma companies competing to bring the first approved NASH drug to market. The growing adoption of digital health platforms and AI-driven patient monitoring solutions is improving trial efficiency, reducing costs, and enhancing data collection accuracy. Additionally, regulatory incentives such as orphan drug designations, fast-track approvals, and breakthrough therapy designations are encouraging drug developers to invest in high-risk, high-reward NASH research. The increasing role of real-world evidence (RWE) in trial designs is further shaping market dynamics, providing valuable insights into long-term treatment outcomes. As clinical research continues to evolve, the NASH clinical trials market is expected to witness rapid growth, driving progress toward effective and scalable treatments for this widespread liver disease.Report Scope
The report analyzes the Non-alcoholic Steatohepatitis Clinical Trials market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase (Phase I, Phase II, Phase III, Phase IV); Study (Interventional Studies, Observational Studies, Expanded Access Studies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$2.0 Billion by 2030 with a CAGR of a 6.8%. The Phase II Clinical Trials segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $828.8 Million in 2024, and China, forecasted to grow at an impressive 5.3% CAGR to reach $697.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Non-alcoholic Steatohepatitis Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Non-alcoholic Steatohepatitis Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Non-alcoholic Steatohepatitis Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABV Technology, AICOOK, BevZero, Breville USA, Cuisinart and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Non-alcoholic Steatohepatitis Clinical Trials market report include:
- 89bio, Inc.
- Akero Therapeutics, Inc.
- Alimentiv Inc.
- Allergan plc
- Boehringer Ingelheim
- Cadila Healthcare Limited
- FOMAT Medical Research
- Gilead Sciences, Inc.
- ICON plc
- Intercept Pharmaceuticals, Inc.
- Laboratory Corporation of America Holdings (LabCorp)
- Madrigal Pharmaceuticals, Inc.
- NGM Biopharmaceuticals, Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Poxel SA
- ProSciento, Inc.
- Viking Therapeutics, Inc.
- Zealand Pharma A/S
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 89bio, Inc.
- Akero Therapeutics, Inc.
- Alimentiv Inc.
- Allergan plc
- Boehringer Ingelheim
- Cadila Healthcare Limited
- FOMAT Medical Research
- Gilead Sciences, Inc.
- ICON plc
- Intercept Pharmaceuticals, Inc.
- Laboratory Corporation of America Holdings (LabCorp)
- Madrigal Pharmaceuticals, Inc.
- NGM Biopharmaceuticals, Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Poxel SA
- ProSciento, Inc.
- Viking Therapeutics, Inc.
- Zealand Pharma A/S
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 144 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
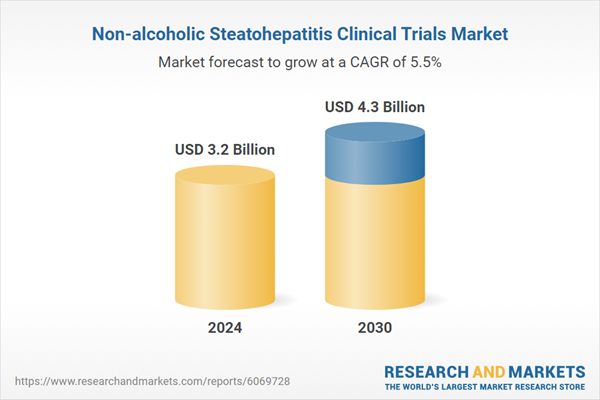
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 4.3 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |