In-Vitro Diagnostics (IVD) Enzymes - Key Trends & Market Drivers Summarized
The in-vitro diagnostics (IVD) enzymes market has become a critical segment within the broader diagnostics industry, providing essential biochemical components for various diagnostic assays and tests. These enzymes are integral to molecular diagnostics, immunoassays, clinical chemistry, and point-of-care testing (POCT), enabling accurate and efficient disease detection. As global healthcare systems emphasize early disease diagnosis and personalized medicine, demand for high-performance IVD enzymes continues to surge. The increasing prevalence of infectious diseases, chronic conditions, and the expansion of decentralized testing facilities are further driving advancements in enzymatic diagnostic solutions.How Are Technological Advancements Improving IVD Enzyme Applications?
Recent technological innovations have significantly enhanced the performance, stability, and specificity of IVD enzymes, driving their widespread adoption across diverse diagnostic applications. One of the most notable advancements is genetic engineering and recombinant enzyme technology, which has allowed for the development of highly stable and efficient enzyme formulations. Recombinant enzymes offer superior consistency, reduced batch-to-batch variation, and improved scalability, making them ideal for large-scale diagnostic production. Additionally, advancements in protein engineering have enabled the customization of enzyme properties to enhance sensitivity and specificity for target analytes.Another key innovation is the integration of nanotechnology in enzyme-based diagnostics. Nanoparticles, such as gold nanoparticles and quantum dots, are being conjugated with enzymes to amplify detection signals, improving assay sensitivity and accuracy. This is particularly beneficial in early-stage disease detection and rapid diagnostic testing. Furthermore, the miniaturization of diagnostic platforms and the development of lab-on-a-chip (LOC) technologies have increased the efficiency of enzyme-based assays, reducing the volume of reagents required and enhancing portability for point-of-care applications.
Automation is also playing a crucial role in the advancement of IVD enzyme applications. The incorporation of robotic liquid handling systems and high-throughput screening techniques in diagnostic laboratories has improved the efficiency and reproducibility of enzyme-based assays. The integration of artificial intelligence (AI) and machine learning (ML) in diagnostic platforms further optimizes data interpretation, allowing for faster and more accurate patient diagnosis. These technological improvements are not only enhancing the precision of in-vitro diagnostic tests but also enabling the development of next-generation diagnostics for personalized medicine.
What Market Trends Are Shaping the Growth of the IVD Enzymes Industry?
Several key trends are reshaping the IVD enzymes market, influencing its growth and adoption across the healthcare sector. One of the most significant trends is the rising demand for molecular diagnostics, particularly in the detection of infectious diseases and genetic disorders. With the increasing need for rapid and highly sensitive diagnostic tools, polymerase chain reaction (PCR) and isothermal amplification technologies have become the gold standard for molecular testing, driving the demand for enzymes such as Taq polymerase, reverse transcriptase, and ligases. The widespread adoption of next-generation sequencing (NGS) and CRISPR-based diagnostic platforms is further accelerating the need for specialized enzymes with enhanced stability and efficiency.Another crucial trend is the expansion of point-of-care testing (POCT) and decentralized diagnostics. The growing preference for portable and rapid diagnostic solutions, particularly in resource-limited settings, has fueled the demand for enzymes used in lateral flow assays, biosensors, and microfluidic devices. Enzymes such as horseradish peroxidase (HRP), glucose oxidase, and alkaline phosphatase are widely used in POCT devices, enabling quick and reliable detection of various biomarkers. The COVID-19 pandemic further accelerated the shift toward decentralized testing, highlighting the importance of rapid enzyme-based assays in pandemic preparedness and response strategies.
The increasing focus on personalized medicine and companion diagnostics is also influencing market trends. As precision medicine gains traction, diagnostic assays tailored to individual genetic profiles and disease markers require highly specific enzymes to detect low-abundance biomarkers. This has led to the development of engineered enzymes with enhanced affinity and activity, ensuring more accurate and individualized diagnostic outcomes. Additionally, the rising geriatric population and the prevalence of chronic diseases, such as cancer, diabetes, and cardiovascular conditions, are driving the demand for enzyme-based diagnostic tests that facilitate early disease detection and monitoring.
What Is Driving the Growth of the IVD Enzymes Market?
The growth in the in-vitro diagnostics enzymes market is driven by several factors, including the increasing prevalence of infectious and chronic diseases, advancements in molecular diagnostics, and the expanding application of enzyme-based assays in point-of-care and decentralized testing. One of the primary growth drivers is the rising demand for high-throughput and automated diagnostic solutions. With healthcare facilities and laboratories seeking faster turnaround times and improved accuracy in test results, the adoption of enzyme-based diagnostic platforms is on the rise.Another major driver is the technological advancements in enzyme engineering and recombinant protein production. The ability to produce enzymes with enhanced stability, efficiency, and specificity has significantly improved the reliability of diagnostic assays, making them more suitable for complex and high-sensitivity applications. Additionally, the increasing adoption of biosensors and wearable diagnostics is creating new opportunities for enzyme-based detection technologies, particularly in continuous glucose monitoring (CGM) and real-time health monitoring.
The expansion of the biopharmaceutical industry and research collaborations between diagnostic companies, academic institutions, and biotechnology firms is also fueling market growth. Increased investments in diagnostic R&D, particularly in the development of novel enzyme formulations for liquid biopsy and digital PCR assays, are driving innovation in the industry. Moreover, the growing regulatory approvals for enzyme-based diagnostic tests and favorable government initiatives promoting early disease detection are further propelling market expansion.
Finally, the rising awareness of disease prevention and early detection strategies among healthcare professionals and consumers is increasing the demand for enzyme-based diagnostic tests for routine health screening and disease monitoring. As diagnostic technologies continue to evolve, the role of IVD enzymes in improving healthcare outcomes and facilitating precision medicine is expected to grow, ensuring a strong market trajectory in the coming years.
Report Scope
The report analyzes the In-Vitro Diagnostics Enzymes market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Enzyme (Proteases, Polymerase & Transcriptase, Ribonuclease, Others); Disease Type (Infectious disease, Diabetes, Oncology, Cardiology, Nephrology, Autoimmune diseases); Technology (Histology Assays, Molecular Diagnostics, Clinical Chemistry); End-Use (Pharma & Biotech, Hospital & Diagnostic Labs, Contract Research Organizations, Academic Labs).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Proteases segment, which is expected to reach US$2.4 Billion by 2030 with a CAGR of a 8.6%. The Polymerase & Transcriptase segment is also set to grow at 10% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $898.7 Million in 2024, and China, forecasted to grow at an impressive 13.7% CAGR to reach $1.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global In-Vitro Diagnostics Enzymes Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global In-Vitro Diagnostics Enzymes Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global In-Vitro Diagnostics Enzymes Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bank of America Securities, Barclays, BMO Capital Markets, BNP Paribas, Cantor Fitzgerald and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this In-Vitro Diagnostics Enzymes market report include:
- Advanced Enzymes Technologies
- Affymetrix
- Aldevron
- Amano Enzyme
- American Laboratories
- BBI Solutions
- Biocatalysts
- Cepheid
- Codexis
- DiaSorin
- EKF Diagnostics
- Eurogentec
- Kaneka
- Merck KGaA
- Promega
- Qiagen
- Roche
- Sanofi
- Seegene
- Thermo Fisher Scientific
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advanced Enzymes Technologies
- Affymetrix
- Aldevron
- Amano Enzyme
- American Laboratories
- BBI Solutions
- Biocatalysts
- Cepheid
- Codexis
- DiaSorin
- EKF Diagnostics
- Eurogentec
- Kaneka
- Merck KGaA
- Promega
- Qiagen
- Roche
- Sanofi
- Seegene
- Thermo Fisher Scientific
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 478 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
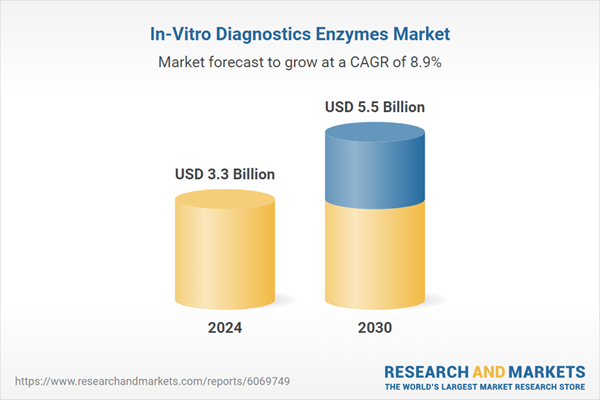
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 5.5 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |