Global Bone Void Fillers Market - Key Trends & Drivers Summarized
What Are the Technological Innovations Enhancing Bone Void Fillers?
Bone void fillers are at the forefront of orthopedic innovation, with technological advancements revolutionizing their formulation, performance, and clinical applications. Recent developments in biomaterials have led to the creation of fillers that combine bioactive ceramics, polymers, and calcium phosphates, which not only fill bone defects but also promote osteoconduction and accelerate natural bone regeneration. Advances in nanotechnology and surface modification techniques are improving the integration of bone void fillers with host tissues, ensuring that the material mimics natural bone structure and mechanical properties. Precision manufacturing processes, such as 3D printing and computer-aided design (CAD), are enabling the customization of filler shapes and sizes to match patient-specific bone defects, thereby optimizing clinical outcomes. Enhanced viscosity and setting properties are being engineered to allow for minimally invasive delivery via injectable systems, reducing surgical trauma and recovery time. The incorporation of growth factors and bioactive molecules into filler formulations is further promoting cellular proliferation and vascularization, essential for successful bone healing. Innovations in degradable materials are ensuring that the filler gradually resorbs as new bone forms, eliminating the need for secondary surgery. Digital imaging and intraoperative navigation systems are enhancing surgical precision by providing real-time feedback on the placement and performance of bone void fillers. These technological advancements are establishing new benchmarks for the efficacy, safety, and ease of use of bone void fillers in a variety of orthopedic applications, from trauma to spinal surgery.How Are Clinical Applications and End-User Demands Shaping the Bone Void Fillers Market?
The clinical landscape for bone void fillers is evolving rapidly, driven by an expanding range of orthopedic applications and the increasing demand for minimally invasive, patient-specific solutions. In trauma and fracture repair, bone void fillers are indispensable in filling defects resulting from comminuted fractures, offering a scaffold for new bone growth and reducing the risk of implant failure. The rising prevalence of degenerative bone diseases, such as osteoporosis, has further fueled the need for effective void fillers that can restore bone strength and stability. In spinal surgeries and joint reconstructions, these fillers are being used to support fusion procedures and augment implant fixation, significantly improving patient outcomes. There is also a growing interest in using bone void fillers in dental and craniofacial applications, where precise, biocompatible materials are required to restore function and aesthetics. As surgeons increasingly adopt digital planning and patient-specific fabrication techniques, the demand for customizable bone void fillers that can be tailored to individual defects is on the rise. Furthermore, the integration of biologics and regenerative medicine principles into filler formulations is transforming clinical practice, allowing for enhanced healing and reduced recovery times. This shift in clinical applications is being driven by an increasing emphasis on personalized medicine, improved surgical techniques, and the growing recognition of the importance of biomimetic materials in orthopedic repair. As a result, the bone void fillers market is witnessing a dynamic shift toward innovative, versatile products that cater to a broad spectrum of orthopedic needs.What Regulatory and Market Dynamics Are Influencing the Bone Void Fillers Industry?
The bone void fillers industry is influenced by a robust regulatory environment and dynamic market forces that drive innovation, quality assurance, and clinical adoption. Regulatory agencies such as the U.S. FDA, EMA, and other international bodies require extensive clinical data, biocompatibility testing, and stringent manufacturing protocols to ensure that bone void fillers meet high standards of safety and efficacy. These regulations necessitate significant investments in research and development, which in turn foster innovation in product design and biomaterial integration. Market dynamics are further shaped by the increasing demand for personalized and minimally invasive orthopedic solutions, prompting manufacturers to explore advanced technologies such as 3D printing and nanofabrication. Strategic partnerships between medical device companies, research institutions, and orthopedic surgeons are accelerating the translation of cutting-edge technologies into commercially viable products. Supply chain considerations, including the availability of high-quality raw materials and advanced processing techniques, are critical for maintaining consistent product performance and regulatory compliance. Additionally, global trends in healthcare expenditure, patient demographics, and clinical outcomes are influencing market strategies and competitive positioning. The emphasis on cost-effective treatments and shorter hospital stays is also driving the adoption of bone void fillers that promote faster healing and reduce the need for additional surgical interventions. These regulatory and market dynamics are fostering a competitive environment where innovation, safety, and clinical performance are paramount, encouraging continuous product improvement and market expansion.The Growth in the Bone Void Fillers Market Is Driven by Several Factors…
The growth in the bone void fillers market is driven by several factors, including technological advancements in biomaterials and personalized manufacturing, an expanding range of clinical applications, and stringent regulatory standards that ensure high product performance and safety. Innovations in bioactive ceramics, biodegradable polymers, and nanotechnology-enhanced formulations are significantly improving the osteoconductive and resorptive properties of bone void fillers. The rising prevalence of orthopedic conditions such as fractures, degenerative bone diseases, and spinal disorders is expanding the end-use applications for these products, while minimally invasive surgical techniques and digital planning are increasing the demand for customizable, patient-specific solutions. Consumer behavior trends in healthcare, driven by an emphasis on improved recovery times, reduced surgical risks, and enhanced long-term outcomes, are further fueling market adoption. Regulatory mandates for clinical efficacy and safety, combined with proactive investments in R&D and strategic collaborations among industry players, are continuously pushing the boundaries of product innovation. These technological, clinical, and regulatory factors, along with the increasing integration of regenerative medicine and digital health platforms, are collectively propelling the bone void fillers market toward sustained global growth and broader clinical acceptance.Report Scope
The report analyzes the Bone Void Fillers market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Material Type (Demineralized Bone Matrix, Calcium Sulfate, Collagen Matrix, Tricalcium Phosphate, Other Material Types); Form (Putty Form, Gel Form, Granules Form, Paste Form, Other Forms); Application (Bone Fracture Application, Spine Fusion Application, Joint Reconstruction Application, Other Applications); End-Use (Hospitals End-Use, Orthopedic Clinics End-Use, Ambulatory Surgical Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Demineralized Bone Matrix segment, which is expected to reach US$1.7 Billion by 2030 with a CAGR of a 6.7%. The Calcium Sulfate segment is also set to grow at 5.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $961.6 Million in 2024, and China, forecasted to grow at an impressive 9.1% CAGR to reach $999.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bone Void Fillers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bone Void Fillers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bone Void Fillers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acumed LLC, Alphatec Spine, Inc. (ATEC), Altimed JSC, Arthrex, Inc., Biopro, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Bone Void Fillers market report include:
- ABYRX, Inc.
- Arthrex, Inc.
- Baxter International, Inc.
- Biocomposites Ltd.
- Bioventus LLC
- ConMed Corporation
- DePuy Synthes
- Exactech, Inc.
- Graftys
- Heraeus Holding GmbH
- Integra LifeSciences Holdings Corporation
- Isto Biologics
- LifeNet Health, Inc.
- Medinova AG
- Medline Industries, LP.
- Medtronic PLC
- Orthofix International N.V.
- Smith & Nephew PLC
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ABYRX, Inc.
- Arthrex, Inc.
- Baxter International, Inc.
- Biocomposites Ltd.
- Bioventus LLC
- ConMed Corporation
- DePuy Synthes
- Exactech, Inc.
- Graftys
- Heraeus Holding GmbH
- Integra LifeSciences Holdings Corporation
- Isto Biologics
- LifeNet Health, Inc.
- Medinova AG
- Medline Industries, LP.
- Medtronic PLC
- Orthofix International N.V.
- Smith & Nephew PLC
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 481 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
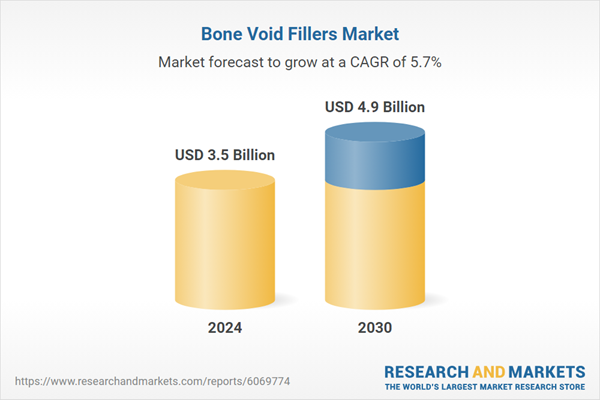
| Estimated Market Value ( USD | $ 3.5 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |