Global Inhaled and Intranasal Products Contract Service Providers Market - Key Trends & Drivers Summarized
What Is Driving the Growth of Contract Services for Inhaled and Intranasal Products?
The market for inhaled and intranasal products contract service providers is experiencing significant expansion, driven by the increasing demand for advanced drug delivery solutions. As pharmaceutical companies seek specialized expertise to develop and manufacture inhalation and nasal drug products, contract service providers (CSPs) have become essential partners in the value chain. The rising prevalence of respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and allergic rhinitis, has spurred the need for efficient pulmonary and nasal drug delivery systems. Additionally, intranasal formulations are gaining traction for systemic drug delivery, particularly in neurological and pain management treatments, further fueling demand for specialized contract manufacturing and development services.The growing complexity of inhaled and intranasal drug formulations necessitates the use of advanced technologies in drug development and delivery. CSPs offer end-to-end solutions, from formulation development to commercial-scale manufacturing, ensuring compliance with stringent regulatory requirements. The shift towards patient-centric drug delivery, including the development of combination products integrating inhalers with digital monitoring systems, is also driving investments in contract services. With pharmaceutical companies focusing on reducing development timelines and operational costs, outsourcing to contract service providers has become a strategic imperative for accelerating product innovation while ensuring quality and regulatory adherence.
How Are Technological Advancements Transforming Inhaled and Intranasal Drug Development?
The inhaled and intranasal drug delivery landscape is undergoing a transformation, fueled by technological innovations that enhance drug formulation, device engineering, and delivery precision. One of the most significant advancements in this space is the development of next-generation inhalation devices, including breath-actuated inhalers, soft-mist inhalers, and digital inhalation systems equipped with sensors for real-time patient monitoring. These technologies improve medication adherence and therapeutic outcomes, creating new opportunities for contract service providers specializing in device formulation and testing. Additionally, advancements in dry powder inhalers (DPIs) and metered-dose inhalers (MDIs) are optimizing drug dispersion and bioavailability, requiring CSPs to integrate cutting-edge analytical techniques in formulation development.Another key technological trend shaping the market is the emergence of intranasal drug delivery for non-respiratory indications. Intranasal formulations are increasingly being explored for central nervous system (CNS) disorders, including migraine, depression, and epilepsy, due to their ability to bypass the blood-brain barrier and provide rapid therapeutic effects. Contract service providers are expanding their expertise in nasal spray formulations, optimizing excipients, and enhancing drug permeation to meet the growing demand for innovative intranasal therapeutics. Moreover, advances in particle engineering, including nanoformulations and liposomal delivery systems, are improving drug solubility and absorption, making inhaled and intranasal drug delivery more efficient and effective. These technological strides are positioning contract service providers at the forefront of innovation, enabling pharmaceutical companies to develop highly specialized drug delivery systems tailored to evolving patient needs.
What Are the Emerging Applications Expanding the Scope of Inhaled and Intranasal Drug Delivery?
The application of inhaled and intranasal drug delivery is expanding beyond traditional respiratory conditions, unlocking new therapeutic possibilities across multiple disease areas. One of the most promising applications is the use of inhaled biologics, including monoclonal antibodies and gene therapies, for treating respiratory and systemic diseases. With advancements in aerosolized biologics, contract service providers are focusing on optimizing particle size, stability, and delivery mechanisms to facilitate the commercialization of inhaled biological therapeutics. This trend is particularly relevant in the treatment of cystic fibrosis, lung infections, and pulmonary fibrosis, where targeted inhalation therapies offer enhanced drug efficacy with minimal systemic exposure.Intranasal drug delivery is also gaining momentum in emergency medicine and pain management, providing a rapid and non-invasive alternative to injectable routes. Nasal sprays for opioid overdose reversal, such as naloxone, have demonstrated the potential of intranasal formulations in critical care settings. Additionally, the development of intranasal vaccines for infectious diseases, including influenza and COVID-19, is accelerating, with contract service providers playing a crucial role in formulation development and scale-up manufacturing. The convenience, fast onset of action, and improved patient compliance associated with nasal drug delivery make it an attractive option for pharmaceutical companies exploring new product pipelines. As research continues to unveil novel applications, CSPs are adapting their capabilities to support the next generation of inhaled and intranasal therapeutics.
What Are the Key Growth Drivers Fueling the Inhaled and Intranasal Products Contract Services Market?
The growth in the inhaled and intranasal products contract service providers market is driven by several factors, primarily centered around technological advancements, regulatory requirements, and increasing demand for specialized drug delivery solutions. One of the most significant growth drivers is the rising incidence of chronic respiratory diseases, which necessitates continuous innovation in inhaled therapies. With COPD, asthma, and pulmonary infections on the rise, pharmaceutical companies are expanding their inhalation product portfolios, creating greater demand for CSPs with expertise in formulation optimization, device development, and regulatory compliance.Another major factor propelling market growth is the increasing adoption of biologics in inhalation and intranasal drug delivery. As pharmaceutical companies explore inhaled biologics and intranasal peptides for systemic diseases, contract service providers are investing in specialized manufacturing capabilities, such as high-precision spray drying and nanoparticle engineering, to support these complex formulations. Additionally, the shift towards personalized medicine is driving demand for customized inhalation devices and patient-specific drug formulations, further reinforcing the need for specialized contract services.
Regulatory advancements and evolving compliance requirements also play a critical role in market expansion. Stringent guidelines from regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are pushing pharmaceutical companies to partner with contract service providers that offer expertise in Good Manufacturing Practices (GMP), stability testing, and regulatory documentation. Furthermore, the increasing focus on environmental sustainability, particularly the transition from chlorofluorocarbon (CFC) propellants to hydrofluoroalkane (HFA)-based MDIs and greener DPI formulations, is driving investments in eco-friendly inhalation technologies. These factors collectively underscore the growing significance of contract service providers in the development and commercialization of inhaled and intranasal drug products, positioning the market for sustained expansion in the coming years.
Report Scope
The report analyzes the Inhaled and Intranasal Products Contract Service Providers market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Delivery (Metered - dose Inhalers, Dry Powder Inhalers, Nebulizers, Others); Services (Quality Assurance, Regulatory Affair Services, Product Design & Development, Product Testing & Sterilization, Product Implementation, Product Upgrade, Product Maintenance, Contract Manufacturing).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Metered - Dose Inhalers segment, which is expected to reach US$3.7 Billion by 2030 with a CAGR of a 7.3%. The Dry Powder Inhalers segment is also set to grow at 7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 11.2% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Inhaled and Intranasal Products Contract Service Providers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Inhaled and Intranasal Products Contract Service Providers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Inhaled and Intranasal Products Contract Service Providers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amneal Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim International GmbH, Chiesi Farmaceutici S.p.A., Cipla Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Inhaled and Intranasal Products Contract Service Providers market report include:
- Aptar Pharma
- Bespak (a Consort Medical company)
- Beximco Pharmaceuticals Ltd.
- Catalent, Inc.
- Colep Healthcare
- DPT Laboratories
- Experic
- Gerresheimer AG
- Grünenthal PRO
- Hovione
- Integral BioSystems LLC
- Intertek Group plc
- Kindeva Drug Delivery
- Lonza Group
- Nano PharmaSolutions, Inc.
- Phillips Medisize (Molex)
- Quotient Sciences
- Recipharm AB
- Summit Biosciences Inc.
- Vectura Group
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aptar Pharma
- Bespak (a Consort Medical company)
- Beximco Pharmaceuticals Ltd.
- Catalent, Inc.
- Colep Healthcare
- DPT Laboratories
- Experic
- Gerresheimer AG
- Grünenthal PRO
- Hovione
- Integral BioSystems LLC
- Intertek Group plc
- Kindeva Drug Delivery
- Lonza Group
- Nano PharmaSolutions, Inc.
- Phillips Medisize (Molex)
- Quotient Sciences
- Recipharm AB
- Summit Biosciences Inc.
- Vectura Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 298 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
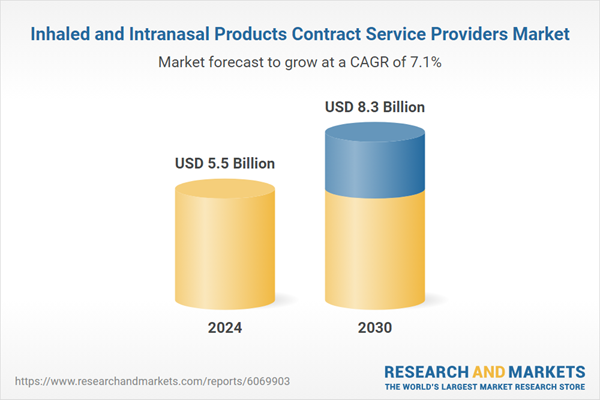
| Estimated Market Value ( USD | $ 5.5 Billion |
| Forecasted Market Value ( USD | $ 8.3 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |