Global Sterile Injectables CDMO Market - Key Trends & Drivers Summarized
Why Are Sterile Injectables CDMOs Emerging as Critical Partners in Modern Biopharmaceutical Manufacturing?
Sterile injectables CDMOs (Contract Development and Manufacturing Organizations) are playing an increasingly strategic role in the pharmaceutical and biotech value chain, as companies seek specialized partners to manage the highly complex, capital-intensive, and strictly regulated production of injectable therapies. With the rise of biologics, vaccines, monoclonal antibodies, and other parenteral drugs, sterile manufacturing capabilities have become a critical bottleneck - one that CDMOs are uniquely equipped to solve through scale, expertise, and end-to-end service offerings. Unlike traditional oral solid dosage forms, sterile injectables demand precision, ultra-clean facilities, and robust quality systems, which require significant investment and technical acumen. As a result, even large pharmaceutical companies are outsourcing sterile injectable production to CDMOs with proven infrastructure, regulatory certifications, and flexible manufacturing capacity. The increased global demand for personalized medicine, biosimilars, and complex formulations is further reinforcing the importance of CDMOs, not only as vendors but as collaborative innovation partners capable of accelerating time-to-market, reducing risk, and navigating regulatory pathways.How Are CDMOs Evolving with Advanced Aseptic Technologies and Integrated Capabilities?
The sterile injectables CDMO market is rapidly advancing, with players adopting cutting-edge technologies, automation systems, and integrated workflows to meet the growing complexity of injectable drug development and commercialization. A key trend is the widespread adoption of isolator-based filling lines and RABS (Restricted Access Barrier Systems), which dramatically reduce contamination risks and enhance operator safety during aseptic processing. Many CDMOs are upgrading their facilities with high-speed, multi-format filling lines that handle vials, prefilled syringes, cartridges, and ampoules - all within the same facility. Integration of single-use systems is another major advancement, reducing cleaning validation burdens and enhancing production agility, especially for biologics and multi-product facilities. Furthermore, CDMOs are increasingly providing full lifecycle services - from early-stage formulation and analytical method development to aseptic fill-finish, lyophilization, labeling, and serialization. Digital technologies such as eBatch records, real-time analytics, and automated quality control systems are also improving process consistency, traceability, and regulatory compliance. With regulators tightening sterility assurance standards globally, CDMOs are proactively investing in GMP upgrades, workforce training, and data integrity systems to stay competitive and serve as long-term partners to clients developing injectable therapies.Where Is Market Demand Accelerating, and Which Segments Are Fueling Expansion?
Demand for sterile injectables CDMO services is accelerating across geographies and therapeutic categories, driven by both established and emerging pharmaceutical markets. North America and Western Europe continue to lead in terms of CDMO contract value, driven by innovation-intensive biotech hubs, well-established regulatory systems, and mature outsourcing strategies among pharmaceutical companies. However, Asia-Pacific - particularly India, China, and South Korea - is witnessing the fastest growth due to expanding biomanufacturing capacity, competitive cost structures, and growing alignment with international quality standards. In terms of therapeutic areas, oncology leads the charge, as most modern cancer therapies are delivered parenterally and require highly specialized sterile processing. Other key growth areas include autoimmune and inflammatory diseases, metabolic disorders like diabetes, and CNS-related conditions - many of which rely on long-acting injectable formulations. The global expansion of vaccine manufacturing, spurred by COVID-19 and future pandemic preparedness efforts, is also fueling demand for high-throughput fill-finish services. Additionally, small- and mid-sized biotech companies - often lacking in-house capabilities - are becoming major clients, engaging CDMOs for not only manufacturing but also regulatory support, clinical trial material production, and market supply strategies.What's Driving the Long-term Growth of the Sterile Injectables CDMO Market Globally?
The growth in the sterile injectables CDMO market is driven by structural shifts in the pharmaceutical industry, evolving therapeutic modalities, global regulatory expectations, and the increasing complexity of parenteral drug development. A core driver is the rise of biologics and specialty injectables, which are difficult and costly to manufacture in-house, creating sustained demand for CDMOs with deep technical expertise and sterile infrastructure. The trend toward leaner, asset-light business models among pharma and biotech companies is reinforcing outsourcing as a strategic necessity rather than an operational choice. Regulatory pressure to maintain data integrity, product traceability, and aseptic precision is also pushing sponsors to partner with CDMOs that can meet compliance across global markets. The growing need for rapid scale-up, flexible capacity, and global supply chain resilience - particularly in the context of emerging infectious diseases and biologic drug launches - has solidified the CDMO's role as a risk-sharing, innovation-enabling partner. As digitalization, personalized medicine, and decentralized trials reshape the pharmaceutical landscape, sterile injectables CDMOs that offer speed, scalability, and regulatory sophistication will remain essential enablers of global healthcare delivery - positioned for sustained, long-term growth.Report Scope
The report analyzes the Sterile Injectables CDMO market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Molecule Type (Small Molecule, Large Molecule); Product (Pre-filled Syringes, Vials & Ampoules, Specialty Injectables, Others); Service (Formulation Development, Analytical & Testing Services, Manufacturing, Packaging, Storage, Others); Administration Route (Subcutaneous, Intravenous, Intramuscular, Others); Therapeutic Area (Oncology, Cardiovascular Diseases, Central Nervous System Diseases, Infectious Disorders, Musculoskeletal Diseases, Hormonal Diseases, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecule segment, which is expected to reach US$3.7 Billion by 2030 with a CAGR of a 5.9%. The Large Molecule segment is also set to grow at 9.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.1 Billion in 2024, and China, forecasted to grow at an impressive 11.3% CAGR to reach $1.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sterile Injectables CDMO Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sterile Injectables CDMO Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sterile Injectables CDMO Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Contract Manufacturing, Aenova Group, Ajinomoto Bio-Pharma Services, Alcami Corporation, Baxter BioPharma Solutions and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Sterile Injectables CDMO market report include:
- AbbVie Contract Manufacturing
- Aenova Group
- Ajinomoto Bio-Pharma Services
- Alcami Corporation
- Baxter International Inc.
- Bespak (Consort Medical)
- Boehringer Ingelheim
- Catalent, Inc.
- Cipla Inc.
- CordenPharma
- Delpharm
- Eurofins CDMO
- Famar Health Care Services
- Fresenius Kabi AG
- Grand River Aseptic Manufacturing
- Grifols S.A.
- Hikma Pharmaceuticals plc
- Jubilant HollisterStier
- Lonza Group
- NextPharma Technologies
- Patheon (Thermo Fisher)
- Pfizer CentreOne
- Recipharm AB
- Samsung Biologics
- Sandoz International GmbH
- Siegfried Holding AG
- Thermo Fisher Scientific
- Unither Pharmaceuticals
- Vetter Pharma
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Contract Manufacturing
- Aenova Group
- Ajinomoto Bio-Pharma Services
- Alcami Corporation
- Baxter International Inc.
- Bespak (Consort Medical)
- Boehringer Ingelheim
- Catalent, Inc.
- Cipla Inc.
- CordenPharma
- Delpharm
- Eurofins CDMO
- Famar Health Care Services
- Fresenius Kabi AG
- Grand River Aseptic Manufacturing
- Grifols S.A.
- Hikma Pharmaceuticals plc
- Jubilant HollisterStier
- Lonza Group
- NextPharma Technologies
- Patheon (Thermo Fisher)
- Pfizer CentreOne
- Recipharm AB
- Samsung Biologics
- Sandoz International GmbH
- Siegfried Holding AG
- Thermo Fisher Scientific
- Unither Pharmaceuticals
- Vetter Pharma
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 586 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
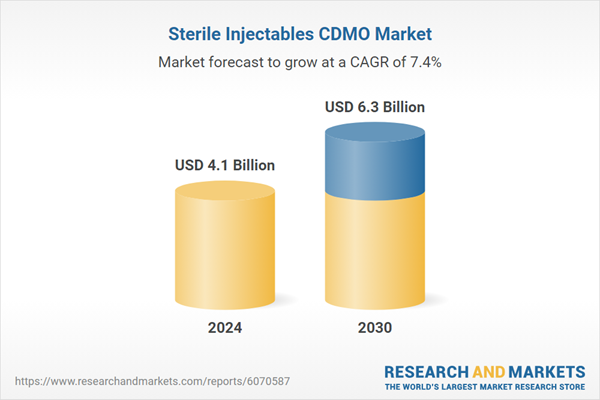
| Estimated Market Value ( USD | $ 4.1 Billion |
| Forecasted Market Value ( USD | $ 6.3 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |