Global Laboratory Products and Services Outsourcing Market - Key Trends & Drivers Summarized
Why Is Outsourcing Becoming a Game-Changer in the Laboratory Industry?
The global laboratory products and services outsourcing market has witnessed a significant surge in demand as laboratories, pharmaceutical companies, and research institutions increasingly turn to external service providers for cost-effective and high-quality testing solutions. Outsourcing has become a key strategy in streamlining operations, optimizing resources, and accelerating drug discovery, clinical research, and diagnostic advancements. The rising complexity of laboratory processes, coupled with stringent regulatory requirements, has prompted organizations to seek specialized expertise from contract research organizations (CROs), contract development and manufacturing organizations (CDMOs), and independent testing laboratories.One of the major drivers of outsourcing in laboratory services is the rising costs associated with in-house laboratory operations. Establishing and maintaining state-of-the-art research facilities requires substantial investments in infrastructure, skilled personnel, equipment, and compliance management. By outsourcing laboratory testing, analytical services, and data processing, organizations can reduce overhead expenses and focus on core research activities. Additionally, outsourcing enables laboratories to access cutting-edge technologies and innovative methodologies without the need for continuous capital expenditure, making it an attractive option for both established pharmaceutical firms and emerging biotech startups.
How Are Technological Advancements Reshaping Laboratory Outsourcing?
The laboratory outsourcing market has benefited immensely from technological innovations, particularly in automation, artificial intelligence (AI), and cloud-based laboratory information management systems (LIMS). Automation has revolutionized sample processing, high-throughput screening, and quality control, enabling contract laboratories to offer faster and more precise testing solutions. The integration of robotic workflows has further enhanced efficiency, reducing human errors and improving data reproducibility. These advancements have made outsourced laboratory services more reliable and cost-effective, encouraging more companies to partner with external providers.AI and big data analytics have also played a transformative role in laboratory outsourcing by streamlining data management, predictive modeling, and regulatory compliance. AI-driven algorithms can analyze large datasets with high accuracy, aiding in biomarker discovery, drug screening, and personalized medicine initiatives. Additionally, the adoption of cloud-based LIMS has improved collaboration between outsourcing partners and clients by enabling real-time data sharing, remote monitoring, and secure access to laboratory reports. These digital advancements have increased the scalability and flexibility of outsourced laboratory operations, allowing organizations to expand their research capabilities without physical infrastructure constraints.
Is the Market Expanding Beyond Pharmaceutical and Biotechnology Sectors?
While pharmaceutical and biotechnology companies have traditionally been the primary users of outsourced laboratory products and services, the market is expanding into diverse industries, including environmental testing, food and beverage safety, forensic analysis, and clinical diagnostics. The increasing need for stringent quality control in consumer goods and industrial products has driven demand for third-party laboratory testing services. Additionally, regulatory authorities worldwide are enforcing more rigorous compliance standards, prompting companies across various sectors to outsource laboratory testing to ensure product safety and regulatory adherence.The rise of precision medicine and genetic testing has further fueled the demand for outsourced laboratory services, particularly in genomic sequencing, personalized drug development, and rare disease diagnostics. Clinical laboratories and healthcare institutions are increasingly partnering with specialized outsourcing firms to access next-generation sequencing (NGS) technologies, molecular diagnostics, and AI-powered predictive analytics. Moreover, the COVID-19 pandemic demonstrated the critical role of outsourced laboratory services in large-scale diagnostic testing, epidemiological studies, and vaccine research, further strengthening the market’ s position.
What Are the Key Growth Drivers Fueling the Laboratory Outsourcing Market?
The growth in the laboratory products and services outsourcing market is driven by several factors, including rising R&D expenditures, increasing demand for cost-efficient laboratory operations, technological advancements, and regulatory pressures. One of the primary growth drivers is the escalating cost of drug development, which has prompted pharmaceutical and biotechnology firms to outsource preclinical and clinical testing to specialized CROs and CDMOs. Outsourcing allows companies to reduce time-to-market for new drugs while maintaining compliance with stringent regulatory frameworks such as FDA, EMA, and ICH guidelines.Another major factor fueling market expansion is the increasing adoption of contract manufacturing for laboratory reagents, assay kits, and consumables. As laboratory workflows become more sophisticated, the need for high-quality, standardized laboratory products has surged, leading to greater reliance on external manufacturers. Additionally, the growing focus on laboratory automation and digitalization has reinforced the role of outsourcing in laboratory informatics, enabling seamless integration of AI-driven analytics, remote diagnostics, and cloud-based data storage.
The expansion of laboratory outsourcing into non-pharmaceutical sectors, such as environmental testing and food safety, has also contributed to market growth. Rising concerns about contamination, pollution, and foodborne diseases have led to stricter testing regulations, increasing demand for third-party laboratory verification. Additionally, the growing emphasis on sustainable and eco-friendly laboratory practices has prompted organizations to outsource testing services to accredited laboratories that specialize in green chemistry, waste reduction, and energy-efficient processes.
Moreover, the globalization of research collaborations and cross-border clinical trials has further driven the need for outsourced laboratory services. As pharmaceutical companies expand their operations into emerging markets, outsourcing provides a cost-effective and scalable solution for conducting clinical research, regulatory compliance testing, and market-specific product evaluations. The ongoing advancements in laboratory technologies, coupled with the increasing demand for specialized expertise, are expected to sustain the robust growth of the laboratory outsourcing market in the coming years.
Report Scope
The report analyzes the Laboratory Products and Services Outsourcing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Laboratory Products Type, Laboratory Services Type); Technology (Immunoassays Technology, Molecular Diagnostics Technology, Microbiology Technology, Clinical Chemistry Technology, Flow Cytometry Technology, Mass Spectroscopy Technology, Chromatography Technology, Other Technologies); End-Use (Pharmaceutical & Biotech Companies End-Use, Medical Device Companies End-Use, CRO & CDMO End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Laboratory Products segment, which is expected to reach US$43.9 Billion by 2030 with a CAGR of a 8.6%. The Laboratory Services segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $11.0 Billion in 2024, and China, forecasted to grow at an impressive 11.8% CAGR to reach $13.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Laboratory Products and Services Outsourcing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Laboratory Products and Services Outsourcing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Laboratory Products and Services Outsourcing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 23andMe, Inc., Abbott, Agilent Technologies Inc., ARUP Laboratories, Bio-Rad Laboratories, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Laboratory Products and Services Outsourcing market report include:
- Agilent Technologies
- Avantor (VWR International)
- Bio-Rad Laboratories
- Charles River Laboratories
- Covance Inc.
- Eurofins Scientific
- ICON plc
- Intertek Group plc
- IQVIA
- Labcorp
- Medpace Holdings, Inc.
- Parexel International Corporation
- PerkinElmer
- PPD, Inc.
- PRA Health Sciences
- Quest Diagnostics
- SGS SA
- Syneos Health
- Thermo Fisher Scientific
- WuXi AppTec, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Agilent Technologies
- Avantor (VWR International)
- Bio-Rad Laboratories
- Charles River Laboratories
- Covance Inc.
- Eurofins Scientific
- ICON plc
- Intertek Group plc
- IQVIA
- Labcorp
- Medpace Holdings, Inc.
- Parexel International Corporation
- PerkinElmer
- PPD, Inc.
- PRA Health Sciences
- Quest Diagnostics
- SGS SA
- Syneos Health
- Thermo Fisher Scientific
- WuXi AppTec, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 389 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
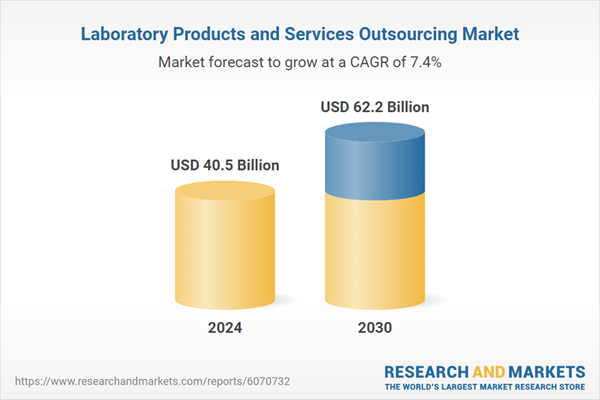
| Estimated Market Value ( USD | $ 40.5 Billion |
| Forecasted Market Value ( USD | $ 62.2 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |