Global High Potency API Contract Manufacturing Market - Key Trends & Drivers Summarized
Why Is High Potency API Contract Manufacturing Crucial for the Pharmaceutical Industry?
High potency active pharmaceutical ingredient (HPAPI) contract manufacturing has become a cornerstone of the pharmaceutical industry, facilitating the production of highly specialized drugs used in oncology, hormone therapies, and targeted therapeutics. HPAPIs are characterized by their potent biological activity at low doses, making them essential for the development of life-saving medications. However, the complexity of handling HPAPIs requires specialized containment facilities, stringent regulatory compliance, and advanced manufacturing capabilities, which many pharmaceutical companies lack in-house. As a result, outsourcing HPAPI production to contract manufacturing organizations (CMOs) has become a strategic move, allowing pharmaceutical firms to focus on research and development while ensuring safe and efficient production. The demand for HPAPI contract manufacturing is further fueled by the growing prevalence of cancer, autoimmune diseases, and neurological disorders, driving the need for potent and precise drug formulations. Additionally, the adoption of targeted drug delivery systems and antibody-drug conjugates (ADCs) is expanding the scope of HPAPI applications, reinforcing the importance of specialized contract manufacturing services.What Technological Innovations Are Shaping the HPAPI Manufacturing Landscape?
The HPAPI contract manufacturing sector is witnessing rapid technological advancements aimed at improving efficiency, safety, and scalability. One of the most significant developments is the integration of high-containment manufacturing facilities equipped with isolators and negative pressure environments, ensuring worker safety and product integrity. Continuous manufacturing technologies are also gaining traction, replacing traditional batch processes with streamlined, automated systems that enhance production efficiency and reduce contamination risks. Additionally, advancements in nanotechnology and particle engineering are revolutionizing HPAPI formulation, enabling better bioavailability and controlled drug release mechanisms. The incorporation of process analytical technology (PAT) and real-time monitoring tools is further optimizing manufacturing operations, ensuring compliance with stringent regulatory guidelines. Furthermore, the use of single-use technologies in HPAPI production is minimizing cross-contamination risks while improving flexibility in multi-product manufacturing environments. As pharmaceutical companies continue to demand higher precision and efficiency in drug manufacturing, contract manufacturers are investing in cutting-edge technologies to stay competitive in the evolving HPAPI landscape.Which Therapeutic Areas Are Driving Demand for HPAPI Contract Manufacturing?
The rising demand for HPAPI contract manufacturing is being driven by the increasing need for highly potent drugs in various therapeutic areas. Oncology remains the dominant sector, with a growing pipeline of targeted cancer therapies, immunotherapies, and ADCs requiring specialized HPAPI manufacturing capabilities. Hormonal treatments, particularly in endocrinology and reproductive health, also rely on HPAPI production to ensure the precise formulation of low-dose but highly effective medications. Neurology is another expanding sector, with drugs for multiple sclerosis, Parkinson’ s disease, and Alzheimer’ s disease requiring potent active ingredients for enhanced therapeutic outcomes. Additionally, the shift toward personalized medicine and precision therapeutics is creating new opportunities for HPAPI manufacturers to cater to specialized drug formulations. The increasing prevalence of rare diseases and orphan drug development is further bolstering demand for HPAPI contract manufacturing, as pharmaceutical companies seek efficient and scalable production solutions for small-batch, high-potency therapies. With evolving treatment paradigms and innovative drug delivery systems, the reliance on contract manufacturers for HPAPI production is expected to intensify across multiple medical domains.What’ s Driving the Growth of the High Potency API Contract Manufacturing Market?
The growth in the high potency API contract manufacturing market is driven by several factors, including the rising incidence of chronic diseases and the increasing complexity of drug formulations. The pharmaceutical industry’ s shift towards targeted therapies and biologics has amplified the demand for HPAPIs, necessitating specialized manufacturing capabilities. Regulatory requirements for handling high potency compounds are also driving pharmaceutical firms to partner with experienced CMOs that possess the necessary infrastructure and expertise. The global expansion of biosimilars and generic HPAPIs is another key driver, as cost-effective alternatives to branded medications gain traction in the market. Additionally, investments in high-containment facilities and advanced manufacturing technologies are improving production scalability and efficiency, further fueling market growth. The growing trend of outsourcing in the pharmaceutical industry, driven by cost optimization and risk mitigation strategies, is also contributing to the expansion of HPAPI contract manufacturing services. With increasing emphasis on innovation, regulatory compliance, and precision medicine, the HPAPI contract manufacturing market is poised for sustained growth, shaping the future of high-potency drug development and commercialization.Report Scope
The report analyzes the High Potency API Contract Manufacturing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Innovative High Potency API Contract Manufacturing, Generic High Potency API Contract Manufacturing); Synthesis (Synthetic Synthesis, Biotech Synthesis); Dosage Form (Injectable Dosage Form, Oral Solids Dosage Form, Creams Dosage Form, Other Dosage Forms); Application (Oncology Application, Hormonal Disorders Application, Glaucoma Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Innovative High Potency API Contract Manufacturing segment, which is expected to reach US$9.6 Billion by 2030 with a CAGR of a 11.9%. The Generic High Potency API Contract Manufacturing segment is also set to grow at 8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.1 Billion in 2024, and China, forecasted to grow at an impressive 14.5% CAGR to reach $3.0 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global High Potency API Contract Manufacturing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global High Potency API Contract Manufacturing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global High Potency API Contract Manufacturing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ADV.1 Wheels, American Racing, Anovia Wheels, BBS Kraftfahrzeugtechnik AG, Breyton Wheels and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this High Potency API Contract Manufacturing market report include:
- AbbVie Contract Manufacturing
- Alcami Corporation
- AMRI (Albany Molecular Research Inc.)
- Aurigene Pharmaceutical Services Ltd.
- Cambrex Corporation
- Carbogen Amcis AG
- Catalent, Inc.
- CordenPharma International
- Curia Global, Inc.
- Dishman Carbogen Amcis Ltd.
- Evonik Industries AG
- Hovione
- Lonza Group AG
- Novasep
- Pfizer CentreOne
- Piramal Pharma Solutions
- Syngene International Limited
- Thermo Fisher Scientific Inc.
- VxP Pharma, Inc.
- WuXi STA (a WuXi AppTec company)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Contract Manufacturing
- Alcami Corporation
- AMRI (Albany Molecular Research Inc.)
- Aurigene Pharmaceutical Services Ltd.
- Cambrex Corporation
- Carbogen Amcis AG
- Catalent, Inc.
- CordenPharma International
- Curia Global, Inc.
- Dishman Carbogen Amcis Ltd.
- Evonik Industries AG
- Hovione
- Lonza Group AG
- Novasep
- Pfizer CentreOne
- Piramal Pharma Solutions
- Syngene International Limited
- Thermo Fisher Scientific Inc.
- VxP Pharma, Inc.
- WuXi STA (a WuXi AppTec company)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 466 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
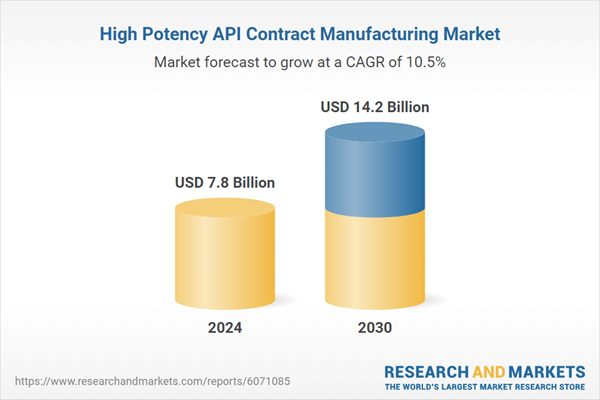
| Estimated Market Value ( USD | $ 7.8 Billion |
| Forecasted Market Value ( USD | $ 14.2 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Global |