Global Unit Dose Manufacturing Market - Key Trends & Drivers Summarized
Is the Shift Toward Precision Dosing Redefining the Pharmaceutical Packaging Landscape?
The rising emphasis on medication safety, dosing accuracy, and regulatory compliance is rapidly propelling the adoption of unit dose manufacturing across pharmaceutical and healthcare sectors. Unit dose packaging involves individually packaged and labeled doses of medications, typically designed for single-use, which enhances patient safety and reduces the risk of medication errors. This format has become especially crucial in hospital and long-term care settings where accurate dispensing plays a critical role in improving treatment outcomes and minimizing adverse drug events. With healthcare systems around the world increasingly embracing lean operations, automation, and digitization, unit dose manufacturing is gaining traction as a cornerstone of efficient medication management. Pharmaceutical companies, contract manufacturing organizations (CMOs), and healthcare institutions are investing in automated unit dose production systems that can handle diverse dosage forms - ranging from oral solids to injectables and inhalables. The format's compatibility with barcode scanning, electronic health records (EHRs), and medication tracking systems further enhances its appeal. As regulatory bodies tighten controls around labeling, traceability, and supply chain transparency, unit dose manufacturing is emerging as an essential component of modern pharmaceutical logistics.Why Are Patient Safety and Hospital Efficiency Fueling the Surge in Unit Dose Packaging?
Hospitals, clinics, and ambulatory care centers are under increasing pressure to reduce medical errors, streamline workflow, and optimize medication administration processes. Unit dose packaging directly supports these goals by providing clearly labeled, tamper-evident, ready-to-use medication units that minimize manual handling by healthcare providers. This is particularly significant in high-volume settings like inpatient wards and emergency rooms, where time-sensitive medication administration and staff workload are major concerns. Unit dose formats also allow healthcare staff to quickly verify correct dosages using barcodes, reducing the potential for cross-contamination or incorrect administration. Moreover, as medication regimens become increasingly personalized and complex, unit dose systems offer the flexibility to tailor treatments to individual patient needs without reliance on bulk packaging or manual preparation. In pediatric and geriatric care - where accurate dosing is especially critical - unit dose solutions improve safety, simplify compliance, and lower risk. Additionally, the unit dose model supports inventory control and waste reduction, offering hospitals a way to manage pharmaceutical costs while maintaining quality of care. These combined benefits are prompting healthcare providers to integrate unit dose formats into both acute and chronic care delivery models, thereby accelerating demand across institutional healthcare settings.Can Automation and Smart Packaging Technologies Transform Unit Dose Manufacturing?
Technological innovation is playing a transformative role in scaling and modernizing unit dose manufacturing capabilities. Automation is at the forefront, with packaging lines now capable of high-speed filling, labeling, sealing, and inspection of individual units with minimal human intervention. Robotic systems and AI-enabled vision inspection tools ensure consistent quality control, while modular production lines allow rapid reconfiguration to accommodate various drug forms and packaging specifications. Smart packaging technologies are also being incorporated into unit dose formats, including RFID tags, QR codes, and time-temperature indicators, which enable real-time tracking and verification throughout the supply chain. These features enhance patient safety, improve inventory visibility, and support compliance with anti-counterfeiting regulations. Digital integration with hospital EHRs and pharmacy management software allows for seamless alignment between prescribing, dispensing, and administration, further reducing errors and increasing efficiency. On the sustainability front, manufacturers are exploring biodegradable films and recyclable materials to align unit dose packaging with environmental goals. These advancements are making unit dose manufacturing more adaptable, efficient, and intelligent - helping pharmaceutical companies and healthcare systems meet evolving expectations for safety, traceability, and operational excellence.What Is Driving the Growth of the Global Unit Dose Manufacturing Market?
The growth in the unit dose manufacturing market is driven by several factors closely tied to technology integration, evolving healthcare practices, regulatory dynamics, and patient safety imperatives. A central growth driver is the global shift toward error-free, automated medication administration in hospitals and long-term care settings, where the need for accurate dosing and traceable dispensing is paramount. The expansion of outpatient and home healthcare services is also fueling demand for convenient, pre-measured, single-use medication formats that reduce complexity for patients and caregivers. Pharmaceutical manufacturers are increasingly partnering with CMOs specializing in unit dose production to meet demand without overburdening internal operations, especially for high-risk and high-volume drugs. Regulatory mandates on drug labeling, serialization, and safety packaging are further accelerating adoption, particularly in North America and Europe. In parallel, technological advances in packaging automation, smart tracking, and data integration are improving scalability and making unit dose manufacturing accessible to a wider range of pharmaceutical firms. Rising focus on cost efficiency and waste reduction in institutional settings is another key factor, with unit dose packaging proving effective in inventory control and resource optimization. As health systems pursue safer, more efficient medication delivery models, these forces are collectively propelling sustained growth in the global unit dose manufacturing market.Report Scope
The report analyzes the Unit Dose Manufacturing market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Sourcing (In-house, Outsourcing); Product (Liquid Unit Dose, Solid Unit Dose, Others); End-Use (Independent Pharmacies, Long Term Care Facility, Hospitals, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the In-house Sourcing segment, which is expected to reach US$81.7 Billion by 2030 with a CAGR of a 8.5%. The Outsourcing segment is also set to grow at 12.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $20.9 Billion in 2024, and China, forecasted to grow at an impressive 13.2% CAGR to reach $27.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Unit Dose Manufacturing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Unit Dose Manufacturing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Unit Dose Manufacturing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Arthrex, Inc., B. Braun Melsungen AG, Boston Scientific Corporation, CONMED Corporation, Endovision Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Unit Dose Manufacturing market report include:
- Advanced Radiant Systems
- Advanced Distributor Products (ADP)
- Airtherm
- Armstrong International Inc.
- Beacon Morris
- Brasch Manufacturing Company, Inc.
- Daikin Industries Ltd.
- Detroit Radiant Products
- Dunham-Bush Limited
- Goodman Manufacturing
- Johnson Controls International Plc
- KING ELECTRICAL MFG. CO
- Kroll Energy GmbH
- Lennox International Inc.
- LG Electronics Inc.
- Marley Engineered Products
- Mitsubishi Electric Corporation
- Modine Manufacturing Company
- New York Blower Company
- Reznor (Nortek Global HVAC)
- Rheem Manufacturing Company
- Trane Technologies plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advanced Radiant Systems
- Advanced Distributor Products (ADP)
- Airtherm
- Armstrong International Inc.
- Beacon Morris
- Brasch Manufacturing Company, Inc.
- Daikin Industries Ltd.
- Detroit Radiant Products
- Dunham-Bush Limited
- Goodman Manufacturing
- Johnson Controls International Plc
- KING ELECTRICAL MFG. CO
- Kroll Energy GmbH
- Lennox International Inc.
- LG Electronics Inc.
- Marley Engineered Products
- Mitsubishi Electric Corporation
- Modine Manufacturing Company
- New York Blower Company
- Reznor (Nortek Global HVAC)
- Rheem Manufacturing Company
- Trane Technologies plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 375 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
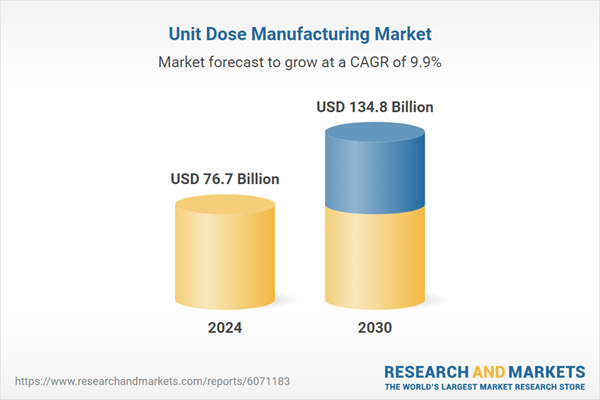
| Estimated Market Value ( USD | $ 76.7 Billion |
| Forecasted Market Value ( USD | $ 134.8 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |