Global Serum-Free Media Market - Key Trends & Drivers Summarized
Is the Shift Toward Defined and Controlled Culture Conditions Accelerating Serum-Free Media Adoption?
The life sciences and biopharmaceutical sectors are undergoing a fundamental shift in cell culture methodologies, and serum-free media (SFM) is emerging as a pivotal solution in response to growing demand for consistency, reproducibility, and safety in cell-based processes. Serum-free media eliminates the use of animal-derived serum, offering a more chemically defined and controlled environment for cell growth, which is crucial in applications where even slight variations in composition can affect outcomes. In biologics manufacturing, especially in the production of monoclonal antibodies, vaccines, and recombinant proteins, SFM is increasingly favored to reduce lot-to-lot variability, minimize the risk of contamination, and simplify downstream purification. Furthermore, academic and commercial research labs are moving toward SFM to meet ethical concerns around animal-derived products and comply with stringent regulatory expectations. Stem cell research, regenerative medicine, and gene therapy are particularly reliant on high-quality serum-free formulations that maintain cell phenotype and viability over time. These trends reflect a broader industry-wide evolution toward high-performance, application-specific media systems designed to meet the technical and regulatory challenges of next-generation therapeutics and advanced cell biology.Are Technological Advances and Customization Creating a New Breed of Culture Media?
The formulation of serum-free media has evolved from a one-size-fits-all solution to a highly tailored and performance-optimized product category. The increasing complexity of cell-based applications has spurred the need for SFM formulations that are cell-type specific, supporting distinct metabolic needs and functional outcomes for CHO cells, hybridomas, HEK293, stem cells, and primary cells, among others. Advances in metabolic profiling, high-throughput screening, and omics technologies have enabled media developers to understand cellular responses at a granular level, informing precise nutrient balancing and additive inclusion. As a result, companies are now offering modular SFM kits that allow users to tweak compositions based on research or production goals. Additionally, the push toward serum-free and xeno-free systems is prompting a convergence of SFM with protein-free and chemically defined media, eliminating not just animal serum but also complex, undefined protein components. Automation in cell culture systems - especially in high-throughput screening platforms and bioreactor-based production - has further fueled demand for SFM that offers predictable performance, minimal sedimentation, and long-term stability under continuous culture conditions. Altogether, these developments have elevated serum-free media from an ethical alternative to a cutting-edge performance enabler.How Are Regulatory Trends and Quality Control Requirements Shaping the Global SFM Market?
Regulatory bodies including the FDA, EMA, and other regional health authorities are increasingly advocating the use of serum-free, animal-component-free media in both clinical and commercial bioprocessing, citing concerns around viral contamination, zoonotic disease transmission, and batch inconsistency. This regulatory momentum is significantly influencing manufacturer preferences, especially in contract development and manufacturing organizations (CDMOs) and biologics producers, who are under pressure to maintain high compliance while scaling up production. In response, media manufacturers are not only improving documentation, traceability, and consistency of their SFM products, but are also introducing Good Manufacturing Practice (GMP)-grade media specifically for clinical and commercial manufacturing. Furthermore, the pharmaceutical industry's increasing reliance on Quality by Design (QbD) principles is reinforcing demand for culture media that are standardized, reproducible, and compatible with risk mitigation strategies. Alongside this, the global expansion of cell therapy and personalized medicine programs - especially in North America, Europe, China, and South Korea - is driving regional demand for certified, high-performance serum-free media. The emphasis on closed, automated systems in bioprocessing also necessitates media that are highly filtered, sterile, and stable, pushing suppliers to invest in innovative packaging, pre-filled single-use systems, and cold-chain logistics optimization.What's Powering the Rapid Growth in the Serum-Free Media Market?
The growth in the serum-free media market is driven by several factors directly linked to technological innovation, evolving end-user requirements, and regulatory developments. First, the increasing scale of biologics manufacturing - particularly monoclonal antibodies, biosimilars, and cell-based vaccines - is accelerating demand for reliable, GMP-grade SFM that supports large-volume, high-efficiency production. Second, the rise of stem cell therapy, CAR-T treatments, and regenerative medicine is fueling the need for specialized, xeno-free serum-free media that support sensitive and rare cell types without compromising viability or function. Third, the global move toward ethical and sustainable research practices is shifting both academic and industrial labs away from fetal bovine serum (FBS), boosting long-term adoption of serum-free alternatives. Fourth, the widespread deployment of automated and high-throughput screening platforms in drug discovery and biotechnology R&D is increasing reliance on media formulations with consistent performance across assays. Fifth, increased regulatory stringency and demand for documentation in clinical trials and therapeutic manufacturing are favoring the use of well-characterized, chemically defined SFM products. Sixth, competitive pressures among contract manufacturers and biotech firms are driving innovation in cost-effective, customized SFM formulations that reduce downstream purification burdens. Finally, regional expansion of biomanufacturing hubs in Asia-Pacific, Eastern Europe, and Latin America is adding to global SFM consumption, especially as governments invest in biotech infrastructure and local production capabilities. Together, these factors are creating a robust and rapidly evolving market landscape for serum-free media across research, clinical, and industrial verticals.Report Scope
The report analyzes the Serum-Free Media market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (CHO Media, HEK 293 Media, BHK Medium, Vero Medium, Stem Cell Medium, Other Serum-free Media); Type (Liquid Media, Semi-solid & Solid Media); Application (Biopharmaceutical Production, Tissue Engineering & Regenerative Medicine); End-Use (Pharma & Biotech Companies, Research & Academic Institutes, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the CHO Media segment, which is expected to reach US$881.4 Million by 2030 with a CAGR of a 11.1%. The HEK 293 Media segment is also set to grow at 15.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $502.2 Million in 2024, and China, forecasted to grow at an impressive 17.5% CAGR to reach $813.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Serum-Free Media Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Serum-Free Media Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Serum-Free Media Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AB Medical, Inc., Becton, Dickinson and Company, Beijing Hanbaihan Medical Devices Co.,LTD, Bio-Rad Laboratories Inc., BIOSIGMA S.r.l. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Serum-Free Media market report include:
- Athena Enzyme Systems
- Biocompare
- BioIVT
- Bio-Techne Corporation
- Biowest
- Capricorn Scientific GmbH
- CellGenix GmbH (Sartorius)
- Corning Incorporated
- Danaher Corporation
- FUJIFILM Irvine Scientific, Inc.
- ImmunoSpot (CTL)
- Innoprot
- InVitria
- InVivo Biosystems
- Lonza Group
- Merck KGaA
- MP Biomedicals
- Neuromics
- PAN-Biotech GmbH
- PeproTech, Inc.
- PromoCell GmbH
- PromoKine (PromoCell)
- R&D Systems (Bio-Techne)
- Sanvitra GmbH
- Sartorius AG
- ScienCell Research Laboratories
- STEMCELL Technologies Inc.
- Thermo Fisher Scientific Inc.
- Xell AG
- ZenBio, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Athena Enzyme Systems
- Biocompare
- BioIVT
- Bio-Techne Corporation
- Biowest
- Capricorn Scientific GmbH
- CellGenix GmbH (Sartorius)
- Corning Incorporated
- Danaher Corporation
- FUJIFILM Irvine Scientific, Inc.
- ImmunoSpot (CTL)
- Innoprot
- InVitria
- InVivo Biosystems
- Lonza Group
- Merck KGaA
- MP Biomedicals
- Neuromics
- PAN-Biotech GmbH
- PeproTech, Inc.
- PromoCell GmbH
- PromoKine (PromoCell)
- R&D Systems (Bio-Techne)
- Sanvitra GmbH
- Sartorius AG
- ScienCell Research Laboratories
- STEMCELL Technologies Inc.
- Thermo Fisher Scientific Inc.
- Xell AG
- ZenBio, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 476 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
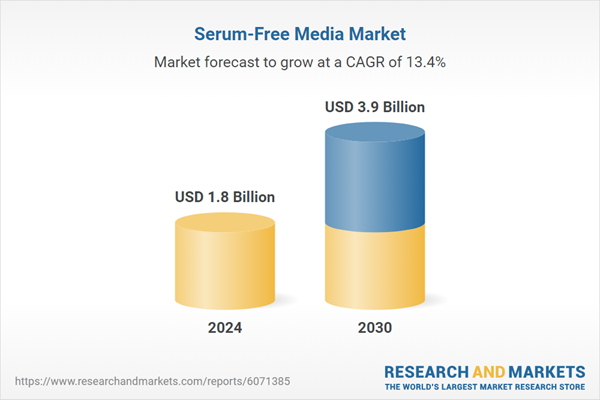
| Estimated Market Value ( USD | $ 1.8 Billion |
| Forecasted Market Value ( USD | $ 3.9 Billion |
| Compound Annual Growth Rate | 13.4% |
| Regions Covered | Global |