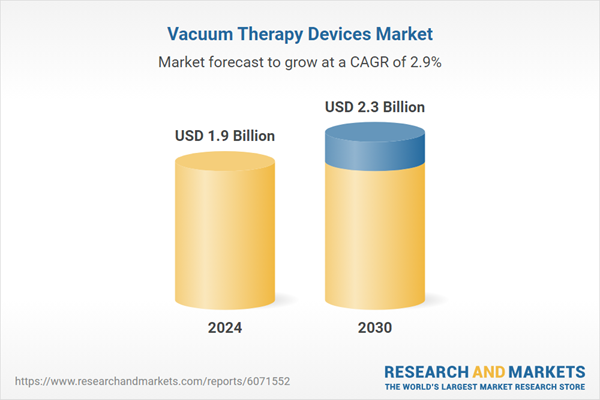
Global Vacuum Therapy Devices Market - Key Trends & Drivers Summarized
Why Is Vacuum Therapy Gaining Ground in Modern Wound Management and Rehabilitation?
Vacuum therapy devices have steadily emerged as a critical innovation in modern therapeutic and clinical practices, particularly in wound care, rehabilitation, and even aesthetic procedures. These devices operate by applying negative pressure to affected tissues, promoting enhanced blood flow, cell regeneration, and wound closure. Their increasing application across a wide spectrum of conditions - from chronic ulcers and post-operative wounds to erectile dysfunction and musculoskeletal injuries - demonstrates their clinical versatility. In wound management, Negative Pressure Wound Therapy (NPWT) has become a mainstay in hospitals and outpatient settings, significantly improving healing rates in diabetic foot ulcers, pressure sores, and surgical wounds. Innovations in dressing materials, portable device designs, and remote monitoring have amplified the effectiveness and patient compliance associated with vacuum therapy treatments. At the same time, clinicians are increasingly integrating vacuum-assisted systems into orthopedic recovery protocols and rehabilitation routines to expedite healing and reduce inflammation. Additionally, there is rising interest in vacuum therapy for cosmetic and dermatological applications such as cellulite reduction and skin toning, especially in aesthetic clinics and wellness centers. This broadening clinical adoption is not only enhancing patient outcomes but is also generating substantial demand for technologically advanced and user-friendly devices that can be tailored for both hospital and home use.How Are Regulatory Shifts and Clinical Guidelines Shaping Adoption Worldwide?
The global landscape for vacuum therapy devices is being strongly influenced by evolving regulatory frameworks, evidence-based clinical guidelines, and health system policy reforms. Regulatory approvals from major authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Japan's PMDA have streamlined device classification and post-market surveillance protocols, ensuring safer and more effective usage. As the evidence base supporting the efficacy of NPWT and other vacuum therapies expands, many healthcare organizations have issued formalized clinical guidelines recommending their use in specific cases, particularly for non-healing wounds and complex trauma injuries. The inclusion of vacuum therapy procedures in national health insurance schemes and reimbursement policies in countries like Germany, Japan, the U.S., and South Korea is significantly accelerating their adoption in public and private healthcare facilities. Simultaneously, regulatory support for digital health integration is encouraging manufacturers to develop vacuum devices embedded with smart sensors, connectivity features, and app-based controls. These features support remote wound monitoring and real-time data analysis, aiding clinicians in customizing treatment and improving patient adherence. Cross-border regulatory harmonization efforts are also opening up new export and licensing opportunities for manufacturers, especially in emerging markets across Asia-Pacific and Latin America. The intersection of rigorous clinical validation, favorable reimbursement frameworks, and digital integration is creating a strong regulatory foundation that is essential for the sustained expansion of vacuum therapy devices worldwide.What Technological Advancements Are Fueling a New Generation of Vacuum Therapy Devices?
Breakthroughs in technology are revolutionizing the vacuum therapy devices market, with a distinct shift toward miniaturization, automation, and intelligent design. One of the most prominent trends is the emergence of compact, lightweight, and battery-operated NPWT systems that provide mobility and convenience for ambulatory and home-based care. These next-generation devices are equipped with features like pressure control automation, moisture sensors, and data transmission capabilities that enhance therapeutic precision and reduce the risk of complications. Material science innovations have led to the development of biocompatible and antimicrobial dressing kits that reduce infection risks and improve patient comfort. Moreover, artificial intelligence (AI) and machine learning (ML) are beginning to influence the space, with predictive analytics being applied to wound healing trajectories and early detection of therapy failures. Telehealth integration is another technological driver, enabling remote consultations, continuous treatment monitoring, and patient education, thereby extending the reach of vacuum therapy beyond traditional clinical environments. Device manufacturers are also investing in modular systems that can be adapted for multiple treatment types, increasing the versatility and return on investment for healthcare providers. Additionally, sustainability and reusability are gaining traction, with a growing number of products now incorporating eco-friendly materials and recyclable components. Collectively, these technological advancements are not only enhancing clinical efficacy but also aligning the market with evolving healthcare delivery models focused on value-based care and patient-centric innovation.What Factors Are Pushing the Boundaries of Growth in the Vacuum Therapy Devices Market?
The growth in the vacuum therapy devices market is driven by several factors directly linked to technological innovation, evolving clinical demands, changing consumer expectations, and expanding healthcare infrastructures. One of the primary drivers is the increasing global burden of chronic wounds, particularly among aging populations and diabetic patients, which has created sustained demand for advanced wound care solutions such as NPWT systems. Additionally, the rising preference for minimally invasive treatments and faster recovery times has propelled the use of vacuum therapy in post-surgical rehabilitation and orthopedic injury management. The expanding availability of home healthcare services and growing demand for patient-managed therapeutic devices have further fueled market penetration of portable vacuum therapy units. On the technological front, the integration of IoT-enabled features and AI-driven analytics has transformed device capabilities, enhancing diagnostic precision and remote treatment customization. Rapid urbanization and lifestyle shifts, especially in middle-income economies, have also led to increased demand for aesthetic vacuum therapies such as body contouring and cellulite reduction. Meanwhile, healthcare providers are investing more in advanced equipment as part of a broader shift towards digital transformation and personalized medicine. Manufacturers are responding with customized device offerings for hospitals, clinics, and home users, supported by robust distribution and training networks. Lastly, increasing awareness among clinicians and patients about the long-term benefits and cost-effectiveness of vacuum therapy, backed by continuous education and promotional efforts by leading brands, is reinforcing the momentum in this highly dynamic and innovation-driven market.Report Scope
The report analyzes the Vacuum Therapy Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Negative Pressure Wound Therapy, Vacuum Constriction Devices); Type (Non-Portable Vacuum Therapy Devices, Portable Vacuum Therapy Devices); Application (Chronic wounds, Acute Wounds, Erectile Dysfunction).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Negative Pressure Wound Therapy segment, which is expected to reach US$1.5 Billion by 2030 with a CAGR of a 3.6%. The Vacuum Constriction Devices segment is also set to grow at 1.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $521.8 Million in 2024, and China, forecasted to grow at an impressive 5.7% CAGR to reach $448.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Vacuum Therapy Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Vacuum Therapy Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Vacuum Therapy Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aptiv PLC, Argus Cyber Security, AUTOCRYPT Co., Ltd., Autotalks Ltd., BlackBerry Certicom and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Vacuum Therapy Devices market report include:
- 3M Company
- ACMD Technology Co., Ltd
- Allied Medical LLC
- Amsino International, Inc.
- ATMOS MedizinTechnik GmbH
- Augusta Medical Systems
- B. Braun Melsungen AG
- Bella Vacuum
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Coloplast Corp.
- ConvaTec Inc.
- Cork Medical
- Drive Medical GmbH & Co. KG
- Kinetic Concepts (3M)
- Laerdal Medical
- Medela AG
- Molnlycke Health Care AB
- Precision Medical, Inc.
- Smith & Nephew plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Company
- ACMD Technology Co., Ltd
- Allied Medical LLC
- Amsino International, Inc.
- ATMOS MedizinTechnik GmbH
- Augusta Medical Systems
- B. Braun Melsungen AG
- Bella Vacuum
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Coloplast Corp.
- ConvaTec Inc.
- Cork Medical
- Drive Medical GmbH & Co. KG
- Kinetic Concepts (3M)
- Laerdal Medical
- Medela AG
- Molnlycke Health Care AB
- Precision Medical, Inc.
- Smith & Nephew plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 361 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.9 Billion |
| Forecasted Market Value ( USD | $ 2.3 Billion |
| Compound Annual Growth Rate | 2.9% |
| Regions Covered | Global |