The Pharmaceutical Membrane Filters Market refers to the global industry focused on membrane-based filtration technologies used in pharmaceutical manufacturing. These filters help in sterilization, contamination control, and product purification processes, ensuring drug safety and regulatory compliance.
The pharmaceutical membrane filters market in North America is segmented into the US, Canada, and Mexico. The North American region is anticipated to play a pivotal role in driving the market's growth. North America, comprising the U.S., Canada, and Mexico, is witnessing significant growth in the pharma membrane filter market due to stringent regulations, rising biologics demand, and expanding manufacturing. The U.S. leads with biotech advancements, Canada sees increased R&D investments, and Mexico benefits from growing pharmaceutical outsourcing, collectively driving market expansion.
In North America, the US holds the highest share of the pharmaceutical membrane filters market. The US pharmaceutical membrane filters market is witnessing robust growth driven by increasing pharmaceutical and biopharmaceutical production, stringent regulatory guidelines for sterile filtration, and rising demand for high-purity filtration solutions. As per Ibis World, in 2024, the United States was home to approximately 2,432 biotechnology companies; likewise, in the contract research organization (CRO) sector, about 4,321 businesses are operating in the US.
Membrane filters play a critical role in ensuring sterility, removing particulates, and improving the drug quality of biologics and biosimilars. Technological advancements, including the development of advanced polymeric membranes and innovations in nanofiltration and ultrafiltration, are fueling the adoption of these filters. Stringent FDA regulations and USP standards necessitate the adoption of high-performance filtration technologies in drug manufacturing, thereby boosting demand for membrane filters across applications such as sterilization, microfiltration, and virus removal.
The growing emphasis on single-use technologies in bioprocessing is driving the adoption of disposable filtration systems, reducing the risk of cross-contamination and enhancing operational efficiency. The rising prevalence of chronic diseases and the subsequent surge in drug development activities, particularly in monoclonal antibodies, vaccines, and recombinant proteins, are contributing to increased consumption of membrane filters. For instance, according to the CDC's Quarterly Provisional Mortality Estimates, heart disease and cancer remain the leading causes of death. For the 12-month period ending in the second quarter of 2024, the age-adjusted death rate for heart disease was 168.5 per 100,000 population, while cancer had a rate of 146.2 per 100,000.
Key players such as Merck Millipore, Sartorius AG, and Danaher Corporation are continuously investing in research and development to enhance filter performance, increase throughput, and comply with evolving industry standards. Strategic collaborations between pharmaceutical manufacturers and filtration technology providers are fostering innovation, enabling the development of customized filtration solutions tailored to specific drug formulations.
The COVID-19 pandemic has underscored the critical role of pharmaceutical membrane filters in vaccine production and therapeutic drug manufacturing. The rising adoption of continuous manufacturing processes in the pharmaceutical sector is also fueling demand for high-efficiency membrane filtration systems that ensure consistent product quality and scalability.
The increasing government and private investments in the pharmaceutical and biotechnology industries are supporting the adoption of high-performance filtration technologies. For instance, in fiscal year 2023, the National Institutes of Health (NIH) received a budget of approximately US$ 47.7 billion, which included US$ 1.412 billion from Public Health Service (PHS) Evaluation financing, US$ 141.5 million in mandatory funding for the Special Type 1 Diabetes account, and US$ 1.085 billion allocated from the 21st Century Cures Act.
The expansion of biopharmaceutical research and increasing clinical trials in the US are expected to sustain the demand for membrane filters, particularly in sterile filtration and cell culture applications. Additionally, the growing focus on sustainability and eco-friendly filtration technologies is encouraging manufacturers to develop energy-efficient and recyclable membrane filtration products.
Advancements in Medical Cable Design to Provide Market trends in Future
The pharmaceutical industry in emerging markets, particularly India and China, is expanding rapidly, transforming these countries into global drug manufacturing hubs. Their competitive advantages - cost-effective production, skilled labor, favorable government policies, and growing domestic demand - are fueling large-scale pharmaceutical production. As a result, the need for advanced membrane filtration systems has surged, ensuring drug purity, regulatory compliance, and manufacturing efficiency.India, often referred to as the "Pharmacy of the World," supplies over 20% of global generic drugs. With more than 3,000 pharmaceutical companies and 10,500 manufacturing facilities, many of which comply with international regulatory standards such as the US FDA and EMA, the country plays a critical role in the global supply chain. China, on the other hand, has made significant strides in biopharmaceuticals and active pharmaceutical ingredients (APIs), with strong government backing and regulatory reforms, such as the Marketing Authorization Holder (MAH) system, which has streamlined the drug manufacturing process. As both nations continue expanding their pharmaceutical exports, the demand for high-quality filtration technologies is growing to ensure compliance with global safety and quality standards.
Given the cost-sensitive nature of manufacturing in these markets, pharmaceutical companies are seeking membrane filtration solutions that balance efficiency with affordability; this has led to increased adoption of high-performance, scalable filtration systems that optimize production costs through reusable or single-use membranes, energy-efficient designs, and high-throughput filtration technologies. Government initiatives such as India’s Production Linked Incentive (PLI) scheme and China’s Made in China 2025 strategy support pharmaceutical self-sufficiency and encourage investments in advanced manufacturing capabilities.
With rising exports to highly regulated markets such as the US and Europe, pharmaceutical firms in India and China must meet stringent filtration standards, further driving demand for cutting-edge membrane filtration solutions. The long-term growth of pharmaceutical manufacturing in these emerging economies is expected to sustain high demand for filtration technologies, supported by increasing healthcare needs, government incentives, and the rise of biosimilars and personalized medicine. As sustainability also becomes a priority, companies are shifting toward eco-friendly filtration systems that reduce waste, lower energy consumption, and enhance operational efficiency.
As India and China solidify their positions as global pharmaceutical powerhouses, advanced membrane filtration technologies will be instrumental in ensuring drug safety, quality, and cost efficiency; this presents a significant opportunity for both domestic and international membrane filter manufacturers looking to expand in these high-growth markets.
Some of the developments are:
To bolster domestic pharmaceutical manufacturing, the Indian government allocated close to US$3 billion under the PLI scheme for pharmaceuticals and medical devices. This initiative successfully attracted investments worth nearly US$4 billion as of April 2024.India's pharmaceutical sector, valued at approximately USD 58 billion, is projected to reach US$ 120-130 billion by 2030 and US$ 400-450 billion by 2047. This growth is driven by factors such as the rising prevalence of lifestyle-related diseases, an aging population, and an increased focus on holistic health.
Between 2022 and 2025, Western pharmaceutical companies increasingly partnered with Chinese firms to access innovative "super me-too" drugs. Major companies such as GSK, Merck, and AstraZeneca entered into billion-dollar agreements to develop and market Chinese drugs internationally, leveraging China's expedited clinical trial processes.sss
In 2023, China and India achieved a record bilateral trade volume of US$136.2 billion, marking a 1.5% increase from the previous year. This growth was supported by a 6% rise in Indian exports to China, indicating strengthening economic ties between the two nations.
The World Health Organization, National Health of Health, and Organization for Economic Co-operation and Development are among the primary and secondary sources referred to while preparing the medical injection molding market report.
Reasons to buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the pharmaceutical membrane filters market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the pharmaceutical membrane filters market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth market trends and outlook coupled with the factors driving the pharmaceutical membrane filters market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Pharmaceutical Membrane Filters Market include:- Sartorius AG
- Parker Hannifin Corporation,
- Asahi Kasei Corporation,
- GEA Group,
- Alfa Laval,
- TAMI Industries,
- Membrane Solutions,
- Koch Industries,
- W L Gore and Associates Inc,
- Meissner Filtration Products, Inc.
- Thermo Fisher Scientific Inc,
- Merck KGaA,
- Danaher Corporation
- 3M.
- Repligen Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 224 |
| Published | March 2025 |
| Forecast Period | 2024 - 2031 |
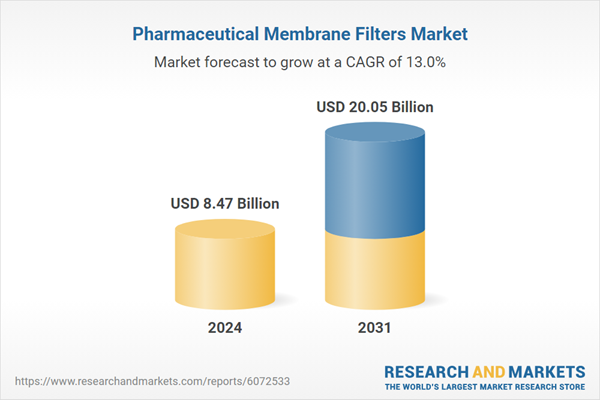
| Estimated Market Value ( USD | $ 8.47 Billion |
| Forecasted Market Value ( USD | $ 20.05 Billion |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |