An escalating introduction of novel clinical trial kits is predicted to boost the market growth during the forecast period. Clinical trial kits play a crucial role in ensuring the consistent, compliant, and effective execution of clinical studies. The design of these kits holds significance in guaranteeing the timely availability of all necessary supplies in a practical and user-friendly manner, aiming to improve both adherence to clinical trial protocols and the ease of use for investigators. These kits are assembled comprehensively to ensure completeness and accessibility, ensuring accurate sample collection, secure and efficient delivery, and inclusion of all necessary documentation. For instance, in February 2023, Gerresheimer unveils its latest Clinical Trial Kit at Pharmapack in Paris. Comprising sterile Gx RTF vials in nest and tub or tray with corresponding closures, this kit is specifically designed to meet the needs of developing new drugs, vaccines, and biologics in the early phases. The Clinical Trial Kit is adaptable for small-batch production, ranging from initial line trials to validation and clinical batches. It is available in six distinct configurations of Gx RTF Glass Vials, and kits featuring Gx Elite and Gx RTF COP vials will be introduced shortly.
By service, logistics was the highest revenue-grossing segment in the global clinical trial kits market in 2023 owing to the expanding size of clinical trials is having a cascading impact on pharmaceutical packaging. There is an increasing emphasis on the design of secondary packaging to minimize the potential for product damage or unintended activation during transportation. Regulatory authorities are urging the pharmaceutical industry to include larger participant cohorts in their studies, leading to a growth in the scale of clinical trials. Additionally, kitting solutions is predicted to grow at the fastest CAGR during the forecast period owing to the increase in the launch of cutting-edge products, encompassing both sample collection kits and medicinal products. The integrity of collecting, storing, processing, and shipping biological samples is crucial for the effective implementation of clinical trials. Several companies providing these services facilitate the conduct of clinical trials by offering tailored lab manuals and kits specific to protocols, streamlining the collection process for companies involved in clinical research. For instance, in October 2022, Q² Solutions, a fully-owned subsidiary of IQVIA and a prominent global provider of clinical trial laboratory services, has introduced the initial self-collection safety lab panel for participants in U.S. clinical trials, making it the first such offering by a leading global clinical trial laboratory.
By phase, phase III was the highest revenue-grossing segment in the global clinical trial kits market in 2023. In comparison to other phases, phase III clinical studies involve a substantial patient population and the application of advanced services to assess the safety and effectiveness of drug candidates. Furthermore, phase III trials are deemed intricate, necessitating robust technologies and dependable clinical resources to efficiently enlist patients, promptly initiate sites, and manage studies cost-effectively. The majority of pharmaceutical, biopharmaceutical, and medical device companies opt to delegate services related to phase III clinical trials to Contract Research Organizations (CROs). Additionally, phase I is predicted to grow at the fastest CAGR during the forecast period. Phase I trials entail a limited participant pool and primarily focus on assessing the safety, optimal dosage, and efficient delivery methods (oral or intravenous) of a drug or treatment. Being the initial stage to test the drug in humans, Phase I trials involve elevated risks, prompting close and thorough monitoring of enrolled individuals. These trials hold significant importance for biotechnology and pharmaceutical firms, serving as a crucial step in evaluating the safety of new drugs in actual patients. For instance, in July 2022, Labcorp, a prominent international life sciences company, has disclosed an enlargement of its automated production line for clinical trial kits in Mechelen, Belgium.
By end-user, pharmaceutical companies was the highest revenue-grossing segment in the global clinical trial kits market in 2023 owing to a rising trend among pharmaceutical firms to delegate their clinical trial operations to specialized Contract Research Organizations (CROs) and research institutions. This strategic move aims to streamline costs, tap into diverse patient pools, harness specialized expertise, and take advantage of the increasing adoption of advanced products. For instance, in October 2023, Cambridge Cognition, a company specializing in the development and promotion of digital tools for evaluating brain health, is excited to introduce AQUA. This automated quality assurance solution is designed for central nervous system (CNS) clinical trials and is driven by the innovative Winterlight speech and language platform developed by the company. Notably, AQUA is the inaugural solution of its kind in the market. Additionally, research institutes is predicted to grow at the fastest CAGR during the forecast period owing to the increase in product innovation, coupled with ongoing advancements in medical technologies like wearable devices, point-of-care diagnostics, and automated sample processing systems, is propelling the creation of cutting-edge clinical trial kits. Manufacturers are prioritizing the integration of these technologies into their kits to enhance efficiency, accuracy, and overall patient experience.
North America region is anticipated for the highest revenue share during the forecast period owing to the positive governmental initiatives, a multitude of companies providing technologically advanced services like at-home clinical trial services, and increasing approvals from regulatory authorities contribute to the favorable conditions. For instance, in May 2022, LabCorp drug development received emergency use authorization from FDA for the first Non-prescription At-Home Collection Kit for combined Covid-19, Flu, and RSV detection. Additionally, Asia Pacific region is predicted to grow at fastest CAGR during the forecast period owing to the expanding pharmaceutical sector, the adoption of supportive government policies, a rise in the establishment of new manufacturing facilities, cost-effectiveness in clinical trials, a swift increase in outsourcing preclinical, clinical, and laboratory testing services, and a heightened emphasis on market leaders' expansion efforts are contributing factors. For instance, in September 2022, Parexel International established a new clinical trial supplies and logistics facility in Suzhou, China. This facility provides both local and international biopharmaceutical clients with quick access to clinical trial materials and medications for sites and patients, thus expediting the progress of clinical trials in the region.
Segmentation: Clinical Trial Kits Market Report 2023 - 2034
Clinical Trial Kits Market Analysis & Forecast by Service 2023 - 2034 (Revenue USD Bn)
- Logistics
- Warehousing & Storage
- Transportation
- Others
- Kitting Solutions
- Sample Collection Kits
- Drugs Kits
Clinical Trial Kits Market Analysis & Forecast by Phase 2023 - 2034 (Revenue USD Bn)
- Phase IV
- Phase III
- Phase II
- Phase I
Clinical Trial Kits Market Analysis & Forecast by End-user 2023 - 2034 (Revenue USD Bn)
- Contract Research Organizations (CROs)
- Pharmaceutical Companies
- Biotechnology Companies
- Research Institutes
- Others
Clinical Trial Kits Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- Charles River Laboratories
- Q2 Solutions
- Almac Group
- Labcorp Drug Development
- Brooks Life Science
- Cerba Research
- Precision Medicine Group
- Clinigen
- Alpha Laboratories
- Labconnect
- Marken
- Patheon (Thermo Fisher Scientific)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2024 |
| Forecast Period | 2023 - 2034 |
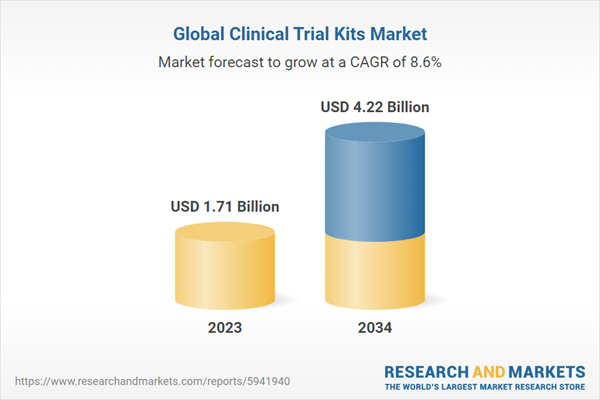
| Estimated Market Value ( USD | $ 1.71 Billion |
| Forecasted Market Value ( USD | $ 4.22 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |