Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
A crucial aspect of GM food safety testing involves the detection and analysis of specific DNA sequences and proteins associated with genetically modified organisms. Various testing methods are used to identify and quantify these genetic elements. Common techniques include polymerase chain reaction (PCR), real-time PCR, and enzyme-linked immunosorbent assays (ELISA). Testing aims to determine whether the newly introduced genes in GM crops may produce allergens that could pose a risk to consumers. This involves assessing the genetic sequences for similarities to known allergenic proteins. Testing is often used to ensure that products are correctly labeled. In many regions, there are strict regulations governing the labeling of GM and non-GM products.
Growing consumer awareness of GM foods and their safety, as well as concerns about potential health and environmental risks, have prompted food producers to invest in rigorous testing to ensure transparency and safety, especially in response to consumer demands. Advances in biotechnology and analytical methods have improved the accuracy, efficiency, and cost-effectiveness of GM food safety testing. These technological advancements have made testing more accessible to a broader range of stakeholders.
In regions where GM and non-GM crops are cultivated in proximity, the risk of cross-contamination is a concern. This necessitates testing to verify compliance with coexistence regulations and ensure product purity. Food manufacturers and producers seek to mitigate the risk of product recalls, legal issues, and reputational damage due to the presence of unapproved GM ingredients in their products. Comprehensive safety testing is a crucial risk management strategy.
Key Market Drivers
Technological Advancements
Polymerase Chain Reaction (PCR) Techniques have seen continuous improvements, such as real-time PCR (qPCR) and multiplex PCR. These techniques allow for the rapid and accurate quantification of specific DNA sequences in GM organisms. Next-Generation Sequencing (NGS) technologies have revolutionized the analysis of genetic material in GM foods. They provide high-throughput sequencing, enabling comprehensive and detailed genetic analysis, including the identification of unknown or unexpected GM elements. Digital PCR (dPCR) is a highly sensitive technique for quantifying target DNA sequences.It offers greater precision and reliability in determining the presence and quantity of GM DNA in a sample. DNA microarrays are used to simultaneously detect multiple GM traits in a single sample. They allow for high-throughput analysis of genetic elements and can identify a wide range of GM components. These point-of-care tests use immunochromatographic techniques to quickly detect the presence of specific GM proteins in a sample. They are user-friendly and provide rapid results, making them suitable for on-site testing.
Key Market Challenges
Sample Handling and Preservation
DNA and proteins, which are commonly targeted in GM food safety testing, are prone to degradation. Inadequate sample handling and preservation can lead to the deterioration of these target molecules, resulting in inaccurate or unreliable test results. Food samples can be complex matrices, containing a wide range of components such as fats, sugars, and enzymes. Improper handling or storage can lead to the degradation of DNA and proteins and may introduce interfering substances that affect the accuracy of testing. Maintaining traceability and proper documentation of sample handling is crucial for regulatory compliance and quality control. Inadequate traceability can lead to questions about the integrity of the sample, especially when it is necessary to retest or confirm results.Cross-contamination between samples or sample handling equipment can lead to false-positive results, especially when testing for trace amounts of GM material. Proper handling procedures are essential to prevent contamination. Sampling variability can occur when collecting samples from large batches or diverse sources. Ensuring consistent and representative sampling is a challenge in GM food safety testing, as it impacts the reliability of test results. Some GM food safety tests require specific sample sizes and quantities, which can be challenging to obtain, particularly when dealing with small or limited samples. Proper storage conditions, including temperature and humidity control, are vital to preserving sample integrity.
Inadequate storage conditions can lead to sample degradation and compromised test accuracy. Samples often need to be transported to testing facilities. The handling and transportation process can introduce additional risks if not executed properly. Ensuring that a sample is well-mixed and homogeneous is essential, especially when testing for GM material that may not be evenly distributed throughout the sample. Inhomogeneity can lead to false-negative results.
Key Market Trends
Organic and Non-GMO Labels
In various countries, regulations and guidelines have been established for the labeling of GM and non-GMO products. To make non-GMO claims, food producers are often required to provide evidence of GM food safety testing to confirm the absence of GM ingredients. The non-GMO and organic labels provide consumers with a clear and easily recognizable way to identify products that meet their preferences. These labels enhance transparency and allow consumers to make informed choices about the food they purchase. Many retailers and food brands have made commitments to offer non-GMO and organic products.To meet these commitments, food manufacturers conduct GM food safety testing to verify the non-GMO status of their products. Using non-GMO and organic labels builds trust between retailers and consumers. These labels reassure consumers that the products they purchase align with their values and dietary preferences. Independent organizations provide non-GMO and organic certifications, often requiring GM food safety testing as part of the verification process. These certifications further support the credibility of non-GMO and organic labels.
Key Market Players
- Bio-Rad Laboratories, Inc.
- Bureau Veritas
- Emsl Analytical Inc.
- Eurofins Scientific SE
- Thermo Fisher Scientific Inc.
- SGS SA
- Intertek Group Plc
- Genetic ID NA Inc.
- ALS Limited
- Assure Quality Limited
Report Scope:
In this report, the Global Genetically Modified Food Safety Testing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Genetically Modified Food Safety Testing Market, By Food Type:
- Crops
- Processed Foods
- Others
Genetically Modified Food Safety Testing Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Genetically Modified Food Safety Testing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Bureau Veritas
- Bio-Rad Laboratories, Inc.
- Emsl Analytical Inc.
- Eurofins Scientific SE
- Thermo Fisher Scientific Inc.
- SGS SA
- Intertek Group Plc
- Genetic ID NA Inc.
- ALS Limited
- Assure Quality Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
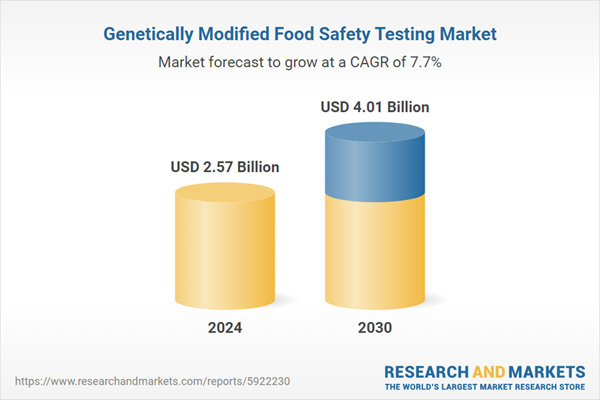
| Estimated Market Value ( USD | $ 2.57 Billion |
| Forecasted Market Value ( USD | $ 4.01 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |