Speak directly to the analyst to clarify any post sales queries you may have.
The cell-free DNA testing market is evolving rapidly, driven by multi-sector collaboration, clinical need for advanced noninvasive diagnostics, and the adoption of data-driven healthcare models. This analysis distills the key opportunities and strategic imperatives shaping industry growth and innovation.
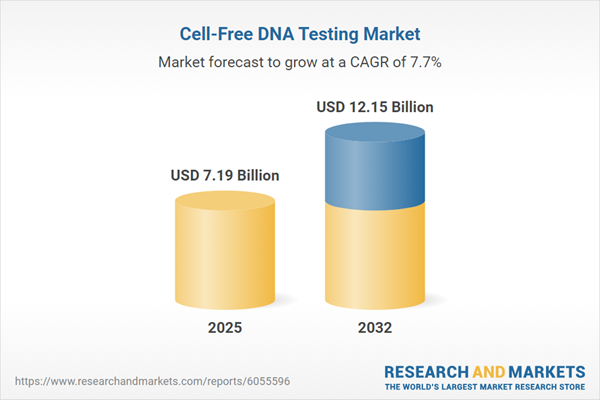
Market Snapshot: Cell-Free DNA Testing Market Growth Overview
The cell-free DNA testing market grew from USD 6.70 billion in 2024 to USD 7.19 billion in 2025, with ongoing expansion projected at a compound annual growth rate (CAGR) of 7.71%, reaching USD 12.15 billion by 2032.
Growing adoption of noninvasive technologies for disease detection, monitoring, and prognosis across key clinical areas, including oncology, prenatal care, infectious diseases, and transplantation, continues to strengthen its market position with senior decision-makers seeking competitive advantage and operational efficiency.Scope & Segmentation of the Cell-Free DNA Testing Market
This research provides sector-wide intelligence on market forces, regulatory shifts, and innovation across all critical segments:
- Offerings: Infectious diseases testing, oncology and cancer management with early detection, screening and minimal residual disease assessment, prenatal screening with non-invasive prenatal testing and preimplantation genetic testing, as well as transplantation testing.
- Technology Platforms: Mass spectrometry, microfluidics for streamlined processing, nucleic acid technology, polymerase chain reaction (PCR), and emerging single-molecule methylation for epigenetic insights.
- End-User Groups: Diagnostic laboratories and testing centers focused on automated, high-throughput solutions; hospitals and clinics prioritizing rapid turnaround and integrated clinician workflows.
- Regions: Americas (North America and Latin America). Europe, Middle East, and Africa (with focused insights into key European, Middle Eastern, and African submarkets). Asia-Pacific (encompassing established and emerging healthcare systems, including China, India, Japan, Australia, and others).
- Key Companies Profiled: Abbott Laboratories, Adaptive Biotechnologies Corporation, Agilent Technologies, Inc., BGI Genomics Co., Ltd., Biodesix, Inc., Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche, Fulgent Genetics, Inc., Guardant Health, Inc., Laboratory Corporation of America Holdings, llumina, Inc., Myriad Genetics, Inc., Natera, Inc., OPKO Health, Inc., PerkinElmer, Inc., Qiagen N.V., Revvity, Inc, SOPHiA GENETICS AG, Stilla Technologies, Thermo Fisher Scientific, Inc.
Key Takeaways: Strategic Insights for Senior Decision-Makers
- Technology advancements in sequencing, bioinformatics, and sample preparation are transforming diagnostic accuracy, expanding the scope and predictive power of liquid biopsy and noninvasive testing applications.
- Shifting regulatory frameworks and payer requirements highlight the growing need for robust clinical utility data, influencing reimbursement and accelerating clinical adoption.
- Partnerships between clinical laboratories, industry innovators, and academic researchers are driving standardization, supporting quality assurance, and unlocking new biomarker-based solutions.
- Distinct end-user requirements—from large-scale diagnostic centers to hospital-based providers—necessitate targeted go-to-market models, pricing strategies, and service differentiation.
- Region-specific factors such as public-private partnerships in Asia-Pacific and adaptive regulatory pathways in Europe create both unique entry barriers and opportunities for tailored market expansion.
- Mergers, acquisitions, and platform investments remain central to competitive strategy, positioning leading players to respond quickly to evolving market and policy dynamics.
Tariff Impact: Understanding the 2025 US Tariff Implications
The introduction of US tariffs in 2025 has led to increased costs for core reagents, instruments, and consumables within the cell-free DNA testing industry. As companies face margin compression, strategic supply chain realignment and domestic sourcing initiatives are emerging. Concurrently, payors demand higher-value clinical data, while providers balance pricing adjustments with test utilization efficiency. Collaboration across the value chain is critical to maintain market accessibility and ongoing innovation amidst these macroeconomic pressures.
Methodology & Data Sources: Ensuring Robust, Actionable Insights
This report employs a rigorous, multi-tiered methodology, utilizing primary literature reviews, financial disclosures, and patent analyses, combined with interviews from experts across the commercial and scientific spectrum. Data triangulation and cross-validation methods underpin accuracy, while scenario planning addresses the resilience of key strategic assumptions.
Why This Report Matters: The Value for Stakeholders
- Delivers data-backed, actionable intelligence to inform market entry, product positioning, and partnership strategy across segments and geographies.
- Enables senior leaders to understand the interplay of regulatory, technological, and economic drivers impacting investment and commercialization decisions.
- Empowers evidence-based planning to drive growth and operational resilience in a dynamic, competitive environment.
Conclusion: Navigating the Future of Cell-Free DNA Testing
The cell-free DNA testing market presents significant opportunities for innovation, collaboration, and sustainable growth as technology, regulatory, and payer landscapes evolve. Strategic alignment and regional adaptation position organizations to unlock market potential and advance precision diagnostics worldwide.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Cell-Free DNA Testing market report include:- Abbott Laboratories
- Adaptive Biotechnologies Corporation
- Agilent Technologies, Inc.
- BGI Genomics Co., Ltd.
- Biodesix, Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche
- Fulgent Genetics, Inc.
- Guardant Health, Inc.
- Laboratory Corporation of America Holdings
- llumina, Inc.
- Myriad Genetics, Inc.
- Natera, Inc.
- OPKO Health, Inc.
- PerkinElmer, Inc.
- Qiagen N.V.
- Revvity, Inc
- SOPHiA GENETICS AG
- Stilla Technologies
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 7.19 Billion |
| Forecasted Market Value ( USD | $ 12.15 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |