The Brazil market dominated the LAMEA Intraosseous Infusion Devices Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $26.4 Million by 2031. The Argentina market is showcasing a CAGR of 9.3% during (2024 - 2031). Additionally, The UAE market would register a CAGR of 8.3% during (2024 - 2031).
Integrating these devices with infusion pump systems allows for precise control and titration of fluid and medication delivery rates, enhancing the efficiency and safety of intraosseous infusion therapy in critical care and anesthesia settings. Integration with infusion pump systems enables healthcare providers to precisely control and titrate fluid and medication delivery rates during intraosseous infusion therapy.
Some of these devices have smart connectivity features, enabling real-time data capture, remote monitoring, and integration with electronic medical records (EMRs) for seamless documentation and clinical decision-making. These devices with smart connectivity can seamlessly integrate with electronic medical record (EMR) systems. This enables automatic data transmission and documentation of infusion parameters directly into the patient's electronic health record. This integration eliminates the need for manual charting or documentation, reducing documentation errors, enhancing data accuracy, and ensuring comprehensive and up-to-date medical records for informed clinical decision-making and continuity of care.
In Saudi Arabia, where the prevalence of CVD-related emergencies such as myocardial infarction, cardiac arrest, and acute heart failure is on the rise, healthcare providers face the challenge of delivering timely and life-saving interventions to patients in critical condition. These devices offer a crucial solution for rapidly accessing the vascular system when traditional peripheral venous access is difficult or impossible, particularly in emergencies where every minute counts. According to the Saudi Arabia government, over 30% of Saudi Arabia's adult population (18 years and older) were at risk of developing CVD. Thirty-five percent of the country's population had cholesterol levels above the advised target, according to numerous national and international multicentric research. Therefore, the rising CVD cases in the LAMEA region will increase demand for these devices in the upcoming years.
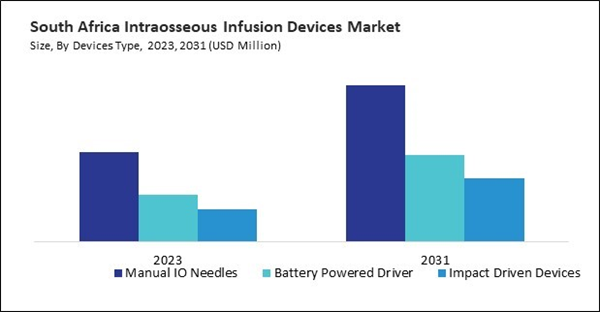
Based on Devices Type, the market is segmented into Manual IO Needles, Battery Powered Driver and Impact Driven Devices. Based on End User, the market is segmented into Hospitals & Clinics, Ambulatory Surgical Centers and Others. Based on countries, the market is segmented into Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria, and Rest of LAMEA.
List of Key Companies Profiled
- Aero Healthcare AU Pty Ltd
- Teleflex Incorporated
- Becton, Dickinson and Company
- BIOPSYBELL S.R.L.
- Cook Medical, Inc.(Cook Group)
- The Seaberg Company Inc. (SAM Medical)
- Argon Medical Devices, Inc. (SHANDONG WEIGAO GROUP MEDICAL POLYMER COMPANY LIMITED)
- Medax Srl
- PAVmed Inc.
Market Report Segmentation
By Devices Type- Manual IO Needles
- Battery Powered Driver
- Impact Driven Devices
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Others
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Aero Healthcare AU Pty Ltd
- Teleflex Incorporated
- Becton, Dickinson and Company
- BIOPSYBELL S.R.L.
- Cook Medical, Inc.(Cook Group)
- The Seaberg Company Inc. (SAM Medical)
- Argon Medical Devices, Inc. (SHANDONG WEIGAO GROUP MEDICAL POLYMER COMPANY LIMITED)
- Medax Srl
- PAVmed Inc.