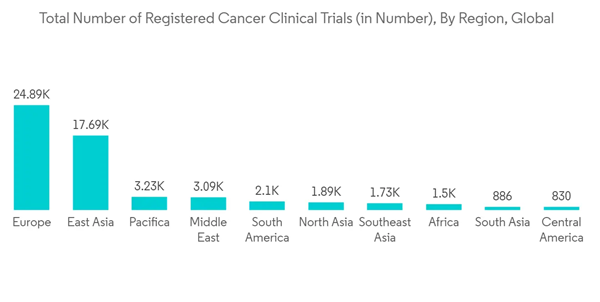
Clinical trials are used in the development and testing of vaccines and other therapeutics; they have been essential in containing the COVID-19 pandemic. For instance, using low-field magnetic resonance imaging, the University of Erlangen-Nürnberg Medical School started a clinical study in August 2021 to assess the prevalence of pulmonary skeletal changes in children and adolescents with verified past SARS-CoV-2 infection (LF-MRI).
Increasing research and development by the key market players during the pandemic is expected to increase market growth during the forecast period. For instance, in August 2021, the government of Canada announced an agreement with leading COVID-19 vaccine developer Moderna, Inc. to build an mRNA vaccine facility in Canada. The goals of the recently unveiled biomanufacturing and life science strategy were in line with Moderna's aspirations to build an mRNA vaccine manufacturing facility in Canada. This is anticipated to drive the clinical trial imaging market growth in Canada, driving the overall growth of the market.
The clinical trial imaging market is expected to grow also due to the rise in the number of contract research organizations, an increase in R&D spending, and growth in pharmaceutical and biotechnological companies. In recent years, various pharmaceutical companies and government organizations have increased their spending on research and development (R&D), which is anticipated to drive the market growth over the forecast period. For example, in FY 2021 Novartis AG, one of the leading participants in the studied market, invested USD 14,886 million, up from USD 14,197 million in the previous year, according to annual reports. Such large investments will provide an impetus to market growth.
Additionally, the market is expected to grow as a result of increased investment in research and development in the biopharmaceutical industry. For instance, the annual research and development expenditure of the biopharmaceutical industry has been 7.3 times greater than that of the aerospace and defense industries, 6.5 times greater than that of the chemicals industry, and 1.5 times greater than that of the software and computer services industry, as per our analysis. Further, as per the IFPMA, in 2022, over 202 billion is estimated to have been spent around the world in biopharmaceutical research and development. The enormous investments in the market are considered a result of the rising acceptance of biopharmaceuticals over the years.
Similarly, increasing contract research organizations are expected to increase the market growth. For instance, in September 2021, Insights Research Organization and Solutions (IROS) - a Contract Research Organization (CRO) in the UAE was launched, specializing in healthcare research and solutions to cover all therapeutic areas.
However, high implementation barriers and costs of imaging systems are expected to hinder market growth.
Clinical Trial Imaging Market Trends
Computed Tomography is Expected to Witness Rapid Growth Over the Forecast Period
Computed tomography is a non-invasive medical procedure that uses X-rays to generate cross-sectional images of the body and provides detailed information for treatment while examining abnormalities in children and adults. Factors such as increasing clinical trials and initiatives by the key players are expected to increase market growth.There are currently 410,319 registered studies, including 61,961 recruiting studies and 169,924 pharmaceutical or biologic studies, dispersed over all 50 states in the US and 220 countries worldwide, according to Clinicaltrials.gov's April 7, 2022, updated figures. As a result, when various types of pharmaceuticals are being created, there will likely be a high need for clinical trial imaging services. Product approval is another factor in market growth. For instance, the Siemens NAEOTOM Alpha device represented a significant technological advance in computed tomography imaging when it received approval from the United States Food and Drug Administration in September 2021. It employs detectors to measure the overall energy present in several x-rays simultaneously. NAEOTOM Alpha measures each x-ray that travels through a patient's body using the cutting-edge CT technology of photon-counting detectors. While minimizing information that is not necessary for the review and analysis, more precise information about the patient can be acquired and used to build images.
Further, acquisitions are one of the key factors behind market expansion, as with acquisitions organizations develop and broaden the scope of the products and services they offer. For instance, MILabs B.V. was acquired by Rigaku Corporation in August 2021. By merging MILabs' multi-modality activities, which include PET, SPECT, optical imaging, and CT, Rigaku extended its life sciences modality business globally. Therefore, it is anticipated that during the predicted time, these acquisitions will contribute to the market's growth.
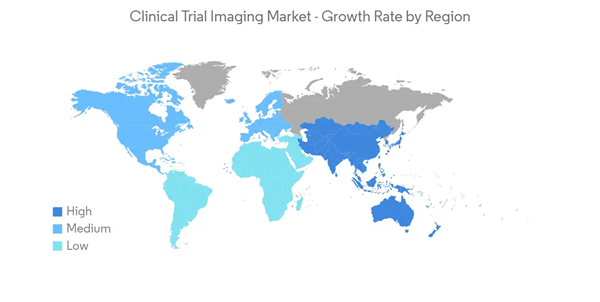
North America is Expected to Dominate the Market Over the Forecast Period
North America is expected to dominate the market during the forecast period. The increasing funding from the United States government for supporting healthcare will contribute to the market's growth in the region. For instance, a 2021 assessment from the Congressional Budget Office found that the National Institutes of Health (NIH) collected more than USD 700 billion in public financing over the previous three decades. Increasing funding is predicted to increase market growth by providing the essential infrastructure to expand clinical trials in the area, increasing the requirement for clinical trial imaging in the area throughout the forecast period.Additionally, according to Pfizer Inc.'s annual report for 2021, the company spent USD 13,829 million on R&D in FY 2021, up significantly from USD 9,393 in the previous year. It is predicted that more products in the company's portfolio will be eligible for clinical studies, which will fuel market expansion in the area. Key companies are expected to expand R&D, which will accelerate market expansion. As of October 2022, "clinicaltrials.gov" reported that 42,240 oncology clinical studies had been registered in the country. As a result, it is predicted that both public and private organizations will increase their spending on research and development over the forecast period.
Similarly, initiatives such as the Canadian government's grant approval to keep conducting cancer studies there are contributing causes to the market's expansion. For instance, the Canadian Cancer Society (CCS) promised the national research network in April 2022 a five-year, USD 30 million investment in support of the Canadian Cancer Trials Group (CCTG). When CCS contributed to the creation of the Canadian Academic Research Group in 1980, it made its largest commitment to research, which the grant extension continues. Such partnerships are anticipated to accelerate market expansion during the anticipated time frame.
Clinical Trial Imaging Industry Overview
The Clinical Trial Imaging Market is moderately competitive and consists of several major players. The key participants of the market studied include Koninklijke Philips N.V, Ixico PLC, Icon PLC, WIRB-Copernicus Group, and Navitas Clinical Research, Inc among others. These major players are undertaking growth strategies such as new product launches, mergers, and acquisitions to retain shares and diversify their product portfolios.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Clario
- Icon PLC
- Ixico PLC
- Koninklijke Philips N.V
- Medpace
- Navitas Clinical Research, Inc
- Parexel International Corporation
- ProScan Imaging
- Radiant Sage LLC
- Resonance Health
- WIRB-Copernicus Group
- Worldcare Clinical LLC