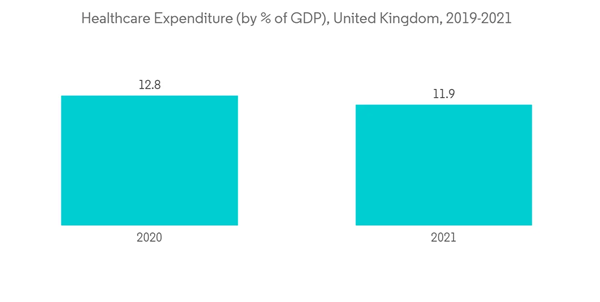
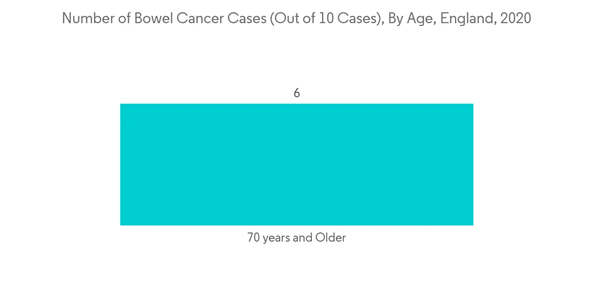
The Europe colorectal cancer screening market is expected to grow at a CAGR of 4.2% during the forecast period.
- The COVID-19 pandemic affected healthcare systems in Europe. It also resulted in the interruption of usual care in many healthcare facilities. The pandemic posed several difficulties for the oncology community, particularly in diagnosing and treating cancer patients while guaranteeing both patient and professional immunity to the virus. A reduction in hospital visits, the discontinuation of the screening programs, and challenges with managing and operating cancer registries were major factors that were affected during the pandemic.
- However, governments across countries in Europe took initiatives to overcome the losses incurred by the pandemic. According to an article published in April 2021 in World Journal of Gastrointestinal Oncology (WJGO), to overcome COVID-19-related disruption, strategies were applied, which included utilizing stool-based cancer tests, developing screening protocols based on individual risk factors, expanding telehealth, and increasing open access colonoscopies.
- As per the source mentioned above, in Europe, a positive fecal immunochemical test (FIT) was recommended, followed up by a colonoscopy. Likewise, strategies and initiatives were adopted across European countries to manage colorectal cancer screening. Thus, these strategies and initiatives during and post-pandemic period in Europe impacted the market significantly. Hence, the market for colorectal screening is witnessing growth over the post-pandemic phase in Europe.
- The advent of efficacious genetic tests, the growing prevalence of colorectal cancer, and increasing cancer prevention initiatives are the primary factors that contribute to market growth.
- Colorectal cancer (CRC) comprises cancer of the colon and rectum. This is one of the most diagnosed forms of cancer and is responsible for severe mortality incidences. According to a press release by DiCE in June 2022, CRC is the 3rd most diagnosed cancer in men and the 2nd in women in Europe, with considerable variability among the 27 countries of the European Union. This shows that CRC creates a high burden on healthcare in Europe. Thus, the burden of CRC establishes a need for cancer screening for better diagnosis and treatment. This is expected to fuel the market growth over the forecast period.
- Additionally to a press release by the EC in March 2022, most European countries conduct screening for colorectal cancer. However, these existing services must be improved and updated to detect more colorectal cancer. This further creates demand for the availability of advanced and innovative devices, new cancer screening strategies, and initiatives, fuelling the market growth.
- Moreover, major players focus more on product development and innovation for CRC screening in the region. For instance, in November 2021, Fujifilm launched ColoAssist PRO, a real-time endoscope visualization system designed to assist endoscopists in performing colonoscopies. ColoAssist PRO is the latest system of Fujifilm's ELUXEO Ultra family of technologies. These innovations in technology and products are anticipated to fuel market growth over the forecast period.
Therefore, due to the above-mentioned factors, the studied market is expected to grow significantly during the study period. However, the high costs associated with screenings are expected to restrain the market growth.
Europe Colorectal Cancer Screening Market Trends
Colonoscopy Segment is Anticipated to Witness Significant Growth Over the Forecast Period
- Compared to other market segments, the colonoscopy segment is expected to witness growth during the forecast period. The colonoscopy procedure is painless and helps remove abnormal tissue before it becomes cancerous. Due to such benefits, colonoscopy is considered the gold standard for colorectal cancer screening. Thus, the segment is expected to witness growth.
- Colonoscopy allows for the detection of early neoplasia and the removal of precancerous lesions, which has been demonstrated to lower the incidence and mortality of CRC significantly. The increase in CRC cases in Europe requires better screening procedures for disease detection and treatment. Thus, increasing awareness regarding colonoscopy and its benefits well known to the public is essential.
- In this context, ECQI Group aims to raise awareness for improved European colonoscopy standards. According to an article published in MDPI in February 2022, when a survey was conducted to evaluate the achievement of the European Society of Gastrointestinal Endoscopy (ESGE) mean withdrawal time (WT) target, most of the samples met the minimum standards set by the ECQI during the colonoscopy procedures. This argues for a better understanding of colonoscopy among the users regarding the same. Thus, this shows that the population of Europe is aware of the need for improved colonoscopy standards across Europe. This awareness is increasing the number of colonoscopy procedures for CRC screening in Europe. Hence, it is expected to fuel the segment's growth over the forecast period.
- Furthermore, having a fecal occult blood test often leads to additional testing. If the fecal occult blood test result is positive, doctors recommend a test such as a colonoscopy to examine the inside of the colon in general. As per an article published in the journal of Gastrointestinal Disorders in June 2022, CRC screening can help reduce the incidence and mortality of the disease. Hence, patients with a positive fecal occult blood test are typically advised to have a colonoscopy for additional testing. Thus, the region's demand for colonoscopies is increasing due to the high burden of CRC in Europe. Thereby, driving the segment growth.
- Hence, the studied segment is expected to witness growth during the forecast period due to the factors mentioned above.
United Kingdom is Anticipated to Contribute Significantly to the Market Growth Over the Forecast Period
- The United Kingdom is expected to significantly contribute to the overall market growth during the forecast period. The growing prevalence of CRC and the increasing number of initiatives for CRC screening are the major factors contributing to the country's market growth.
- Bowel cancer is also known as colorectal cancer, and it affects the large bowel, which is made up of the colon and rectum. With the increasing burden of CRC in the United Kingdom, the demand for the availability of cancer screening in the country is also increasing.
- The 2022 update by Bower Cancer UK shows that bowel cancer is the 4th most common cancer, and approximately 43,000 people are diagnosed with bowel cancer every year in the United Kingdom. This creates a huge healthcare burden in the country and creates demand for screening for early detection of the disease. This is further expected to fuel the market growth in the United Kingdom.
- Furthermore, the United Kingdom's government is more focused on providing better diagnosis and treatment options to the target population and controlling the disease burden. Thus, together with the NHS foundation, the country's government offers bowel cancer screening for people aged 60 to 74 every two years.
- Furthermore, according to the data updated by the government of the United Kingdom in its press release in July 2022, NHS is gradually extending this age range, and people aged 56 or more are now being invited as part of this CRC screening process. In addition, the data updated by the Nuffield Trust in June 2022 stated that there are national screening programs for breast, cervical, and bowel cancer in the United Kingdom, and these services save nearly 9,000 lives every year. Thus, such initiatives in the country propel colorectal cancer screening and drive overall market growth.
- Thus, the market is expected to witness a high growth rate over the forecast period due to the aforementioned factors in the United Kingdom.
Europe Colorectal Cancer Screening Market Competitor Analysis
The Europe colorectal cancer screening market is competitive and consists of several significant players across the region. The key players are developing novel products to compete with the existing products, while others are acquiring and partnering with the other companies trending in the market to expand their presence. Some market players in the studied market are Abbott Laboratories, Epigenomics Inc., Exact Sciences Corporation, F. Hoffmann-La Roche AG, Novigenix SA, Quidel Corporation, Siemens Healthineers AG, Sysmex Corporation, Olympus Corporation, Stryker, and FUJIFILM Corporation.
Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Epigenomics Inc.
- Exact Sciences Corporation
- F. Hoffmann-La Roche AG
- Novigenix SA
- Quidel Corporation
- Siemens Healthineers AG
- Sysmex Corporation
- Olympus Corporation
- Stryker
- FUJIFILM Corporation