The COVID-19 pandemic impacted the growth of the hunter syndrome market. For instance, according to a study published in BMC, in September 2021, lysosomal storage diseases (LSDs), including mucopolysaccharidosis II (MPS II, hunter syndrome), and others have shown that COVID-19 negatively impacted LSD patients' access to continuous care and therapy. The risk of getting COVID-19 was higher in patients with LSDs than in the general population. As per a study published in BMC, in October 2021, about 20% of patients receiving enzyme replacement therapy (ERT) in a hospital setting experienced treatment disruptions due to the COVID-19 pandemic. Thus, the delayed treatment services impacted the market's growth during the pandemic. However, the market started to recover in the later phases of the pandemic, which was reflected in the rising number of product developments and product approvals. For instance, in January 2021, GC Pharma received Japanese manufacturing and marketing approval for a Hunterase ICV (intracerebroventricular) injection of 15 mg as a treatment for mucopolysaccharidosis type II (hunter syndrome).
Certain factors that are attributing to the market's growth are increasing government initiatives, the introduction of novel therapies, and robust pipelines for treating hunter syndrome.
The rising burden of hunter diseases and the increasing patient population led to a rise in R&D and drug discovery, which is expected to increase the market's growth over the forecast period. For instance, in September 2021, Takeda Pharmaceuticals entered an exclusive collaboration and license agreement with JCR Pharmaceuticals to commercialize JR-141, an investigational next-generation recombinant fusion protein of an antibody against the human transferrin receptor and iduronate-2-sulfatase (IDS) enzyme for the treatment of Hunter syndrome. Under the terms of the agreement, Takeda commercialized JR-141 outside of the United States, including Canada, Europe, and other regions (excluding Japan and certain other Asia-Pacific countries). JCR received an upfront payment for such an ex-US license and is eligible to receive additional development and commercial milestones, as well as tiered royalties on potential sales. Such developments are expected to increase the availability of drugs and therapies in the market, thus boosting its growth over the forecast period.
The growing focus on conducting various R&D activities by companies to launch new products for treating hunter diseases is also contributing to the market's growth. For instance, in September 2022, ROBIO Inc. announced that the UK MHRA, Research Ethics Committee (REC), and Health Research Authority (HRA) approved AVROBIO's collaborators for the initiation of the Phase 1/2 clinical trial of investigational autologous hematopoietic stem cell (HSC) gene therapy in infants diagnosed with neuronopathic mucopolysaccharidosis/hunter disease.
The increasing government and organization focus on adopting various strategies such as collaborations and partnerships for developing effective drugs and treatment options are contributing to the growth of the market. For instance, in June 2022, the National MPS Society and Genetic Alliance partnered with Luna to accelerate the development of therapies for treating hunter syndrome in patients. In December 2021, the National MPS Society, Luna, and Genetic Alliance launched a digital drug discovery community in partnership with Takeda Pharmaceutical Company Limited to advance the development of therapeutic interventions for patients with mucopolysaccharidosis type II (MPS II), also known as hunter syndrome.
Therefore, owing to such developments, the market is expected to grow over the forecast period. However, the high treatment costs are likely to impede the market's growth over the forecast period.
Key Market Trends
The Enzyme Replacement Therapy (ERT) Segment is Expected to Witness Significant Growth in the Hunter Syndrome Treatment Market During the Forecast Period
The enzyme replacement therapy is expected to witness significant growth in the hunter syndrome treatment market over the forecast period due to the rising availability of enzyme replacement therapies, increasing awareness, and less stringent guidelines for rare diseases.Enzyme replacement therapy (ERT) is a standard of care for several types of rare diseases and consists of replacing the deficient or absent enzyme with a functional recombinant version through intravenous administration. For instance, as per an article published in October 2021, it has been observed that pabinafusp alfa was effective against both the somatic and CNS symptoms of patients with MPS-II as it can pass through the blood-brain barrier to reach the central nervous tissues. Thus, the high efficacy and safety of the novel enzyme drug are expected to increase its adoption for treating patients with hunter syndrome, thereby propelling the market's growth.
As per an article published in PLOS One, in May 2021, it has been observed that enzyme replacement therapy (ERT) is the primary treatment for mucopolysaccharidosis type-II (hunter syndrome) that helps in improving the course of diseases by reducing the somatic symptoms, including hepatomegaly and splenomegaly. In addition, as per the same source, ERT using human recombinant iduronate 2-sulphatase administered intravenously is considered the current standard of care for patients with hunter syndrome. Thus, such studies are expected to increase the demand for ERT and propel the segment's growth over the forecast period.
The growing development of enzyme replacement therapy products by companies are also likely to increase the availability of ERT therapies in the market, further augmenting its growth. For instance, in March 2021, the MHLW approved IZCARGO (pabinafusp alfa 10 mL, intravenous drip infusion) for the treatment of MPS II in Japan. It is a recombinant iduronate-2-sulfatase enzyme replacement therapy (ERT) developed using J-Brain Cargo, a proprietary technology developed by JCR, to deliver therapeutics across the blood-brain barrier (BBB).
Thus, owing to the high efficacy and safety of ERT, the rising number of research studies, and increasing product approvals, the studied segment is expected to grow over the forecast period.
North America is Expected to Hold a Significant Share in the Hunter Syndrome Treatment Market During the Forecast Period
North America is expected to witness healthy growth over the forecast period due to the rising awareness regarding rare diseases, favorable regulations for orphan drug development, growing healthcare expenditure, and favorable reimbursement policies for expensive drugs in the region. The presence of well-established healthcare infrastructure, increasing adoption of novel therapies, and technological advancements are also expected to fuel the hunter syndrome treatment market.The increasing focus on developing advanced products by companies and rising product launches and approvals in the region are expected to boost the market over the forecast period. For instance, in August 2022, REGENXBIO Inc. announced its plans to file a Biologics License Application (BLA) in 2024 using the FDA's accelerated approval pathway for RGX-121 for the treatment of mucopolysaccharidosis Type II (MPS II). In February 2022, Homology Medicines, Inc. presented data on HMI-203 gene therapy candidate for the treatment of hunter syndrome (MPS II), under phase I dose-escalation clinical study in adults with hunter syndrome, at the Annual WorldSymposium Meeting.
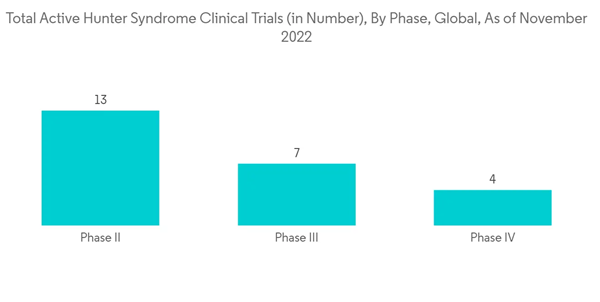
The increasing focus of key players in conducting clinical trials for evaluating the safety and effectiveness of enzyme drugs and therapies for treating patients with hunter syndrome may also contribute to the market's growth. For instance, as per Clinicaltrials.gov, as of September 2022, REGENXBIO Inc. was conducting a phase II/III clinical study to determine the safety and efficacy of RGX-121, a gene therapy, in children aged five and over with severe MPS II. As per the same source, 23 active clinical studies for hunter syndrome are ongoing in the United States, out of which nine are in phase I clinical trials, 10 in phase II, three in phase III, and one in phase IV. Hence, the high number of clinical trials in the country may boost the growth of the hunter syndrome treatment market over the forecast period.
The rising focus on developing various safe treatment options for patients with hunter syndrome and increasing product launches in the country are expected to boost the market's growth during the forecast period.
Competitive Landscape
The hunter syndrome treatment market is moderately competitive and consists of several major players. The companies are focusing on adopting various key strategies such as collaborations, partnerships, product launches, product approvals, and other strategies to withhold their market positions. Some of the companies currently dominating the market are REGENXBIO Inc., Takeda Pharmaceutical Company Limited, Clinigen Group PLC, JCR Pharmaceuticals, ArmaGen, Sangamo Therapeutics, Denali Therapeutics Inc., Bioasis Technologies Inc., Inventiva, etc.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- REGENXBIO Inc.
- Takeda Pharmaceutical Company Limited
- Triley Bidco Limited (Clinigen Group PLC)
- JCR Pharmaceuticals
- Sangamo Therapeutics
- Denali Therapeutics Inc.
- Bioasis Technologies Inc.
- Inventiva
- GC Pharma (Green Cross Holdings)
- Esteve
- Avrobio Inc.
- CANbridge Life Sciences Ltd