Global Upstream Bioprocessing Market - Key Trends & Drivers Summarized
What Is Upstream Bioprocessing, and Why Is It So Crucial in Biopharmaceutical Manufacturing?
Upstream Bioprocessing refers to the initial phase of biomanufacturing, involving the development and cultivation of living cells to produce biologically active substances. This process encompasses activities such as cell line development, media preparation, cell culture, fermentation, and harvest. It is the foundation of biopharmaceutical production, enabling the synthesis of key biological products, including monoclonal antibodies, vaccines, recombinant proteins, and cell and gene therapies. Upstream bioprocessing relies on precise control of conditions like temperature, pH, oxygen, and nutrient levels to optimize cell growth and maximize product yield and quality. Bioreactors, culture media, sensors, and control systems are central components of upstream bioprocessing, ensuring that the biological processes are scalable, efficient, and reproducible.The importance of upstream bioprocessing lies in its role in determining the yield, purity, and efficacy of biopharmaceutical products. It is a critical step in ensuring the overall success of biomanufacturing, as any issues in upstream processes can directly impact downstream purification and final product quality. With the growing demand for biologics, such as antibody-based therapies, vaccines, and next-generation cell and gene therapies, efficient upstream bioprocessing has become more important than ever. As biopharmaceutical companies aim to accelerate drug development timelines and expand production capacity, optimizing upstream processes is key to improving scalability, reducing costs, and increasing the speed of getting therapies to market.
How Are Technological Advancements Shaping the Upstream Bioprocessing Market?
Technological advancements have significantly improved the efficiency, scalability, and automation of Upstream Bioprocessing, driving innovation across the biopharmaceutical sector. One major development is the use of single-use bioreactors, which have revolutionized biomanufacturing by reducing the risk of contamination, lowering capital costs, and increasing flexibility in production. Single-use systems, including bioreactors, mixers, and connectors, are now widely adopted in both small-scale research and large-scale commercial production. These systems enable faster setup, reduced cleaning requirements, and quicker batch turnaround, making them ideal for producing personalized medicines, such as cell therapies and niche biologics.Advancements in cell culture technologies, such as the development of high-performance cell lines and optimized media formulations, have improved cell viability, growth rates, and product yield. The introduction of perfusion systems, which continuously feed cells with fresh nutrients while removing waste, has further increased the efficiency of cell cultures, especially in continuous biomanufacturing. Automation and digitalization are also playing a transformative role in upstream bioprocessing. AI-driven software, advanced sensors, and process analytical technology (PAT) have enabled real-time monitoring and control of bioprocess parameters, ensuring optimal growth conditions and higher reproducibility. The use of data analytics and machine learning models helps predict cell behavior, optimize media composition, and prevent batch failures, enhancing overall productivity. These technological innovations are making upstream bioprocessing faster, more efficient, and adaptable to the evolving needs of biologics production.
What Are the Emerging Applications of Upstream Bioprocessing Across Biopharma Production?
Upstream Bioprocessing is expanding its applications across various segments of biopharmaceutical production, driven by the growing demand for diverse biologics and personalized medicine. In the production of monoclonal antibodies, upstream bioprocessing plays a crucial role in cultivating mammalian cells, such as CHO (Chinese Hamster Ovary) cells, which are the most commonly used host cells due to their ability to produce high-quality proteins. Optimized upstream processes ensure high titers of monoclonal antibodies, improving yield and reducing production costs. In vaccine manufacturing, upstream bioprocessing is essential for cultivating viral vectors, bacterial cultures, or yeast cells, depending on the type of vaccine being produced. The use of advanced bioreactor systems and high-efficiency cell lines has enabled faster and more reliable production of vaccines, including mRNA and viral vector-based vaccines, which were critical during the COVID-19 pandemic.The field of cell and gene therapy is another significant area where upstream bioprocessing is critical. For cell therapies, efficient cell expansion in bioreactors is necessary to generate the large number of cells required for patient treatments. Similarly, in gene therapy, upstream processes involve the cultivation of host cells to produce viral vectors used in gene delivery. High-throughput upstream systems are essential for scaling up production to meet clinical and commercial demands, especially as more gene therapies receive regulatory approval. Upstream bioprocessing is also expanding into biosimilar production, where optimized cell culture processes help achieve cost-effective manufacturing of biologics that are similar to reference products. The emerging applications of upstream bioprocessing in these areas reflect its vital role in supporting the growing biopharmaceutical pipeline and ensuring a steady supply of innovative therapies.
What Drives Growth in the Upstream Bioprocessing Market?
The growth in the Upstream Bioprocessing market is driven by several factors, including the increasing demand for biologics, advancements in biomanufacturing technologies, and growing investments in biotechnology and personalized medicine. One of the primary growth drivers is the rising demand for biologics, such as monoclonal antibodies, vaccines, and recombinant proteins, which require efficient and scalable upstream processes for production. The COVID-19 pandemic accelerated the need for rapid vaccine development and production, highlighting the critical role of upstream bioprocessing in scaling up vaccine manufacturing. The ongoing demand for personalized medicines, including cell and gene therapies, has further fueled investments in upstream technologies that support high cell yields and rapid production cycles.Advancements in single-use technologies, automation, and continuous bioprocessing have also propelled the adoption of upstream bioprocessing solutions across biopharmaceutical companies. Single-use bioreactors, in particular, have reduced infrastructure costs, minimized contamination risks, and provided greater flexibility in switching between different production lines, making them highly suitable for personalized therapies and clinical manufacturing. Regulatory support for biosimilar development has encouraged biopharma companies to optimize upstream processes to achieve cost-effective production, driving growth in the biosimilars segment. Additionally, the integration of AI, digital twins, and process analytical technology (PAT) has improved process control, quality, and efficiency, making upstream bioprocessing more attractive for both small biotech firms and large pharmaceutical manufacturers.
The global focus on expanding biomanufacturing capacity, improving therapeutic access, and achieving cost-effective production has further spurred investments in upstream bioprocessing technologies. As more biologics move through clinical trials and receive regulatory approvals, the demand for robust and scalable upstream bioprocessing solutions is expected to grow. With continuous innovations in bioreactor design, cell culture optimization, and digitalization, the upstream bioprocessing market is poised for sustained growth, supported by the global drive toward more efficient, scalable, and flexible biomanufacturing processes.
Report Scope
The report analyzes the Upstream Bioprocessing market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Workflow (Cell Culture, Media Preparation, Cell Separation); Use Type (Multi, Single).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cell Culture Workflow segment, which is expected to reach US$13.6 Billion by 2030 with a CAGR of a 13.1%. The Media Preparation Workflow segment is also set to grow at 12% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.8 Billion in 2024, and China, forecasted to grow at an impressive 12.1% CAGR to reach $4.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Upstream Bioprocessing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Upstream Bioprocessing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Upstream Bioprocessing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AGC Biologics, Applikon Biotechnology, Biotech Labs Pvt. Ltd., Boehringer Ingelheim International GmbH, CellGenix GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Upstream Bioprocessing market report include:
- AGC Biologics
- Applikon Biotechnology
- Biotech Labs Pvt. Ltd.
- Boehringer Ingelheim International GmbH
- CellGenix GmbH
- Corning, Inc.
- Danaher
- Eppendorf AG
- GE Healthcare
- Lonza Group AG
- Merck KGaA
- Patheon N.V
- PBS Biotech, Inc.
- Samsung Biologics
- Sartorius AG
- Thermo Fisher Scientific, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AGC Biologics
- Applikon Biotechnology
- Biotech Labs Pvt. Ltd.
- Boehringer Ingelheim International GmbH
- CellGenix GmbH
- Corning, Inc.
- Danaher
- Eppendorf AG
- GE Healthcare
- Lonza Group AG
- Merck KGaA
- Patheon N.V
- PBS Biotech, Inc.
- Samsung Biologics
- Sartorius AG
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 166 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
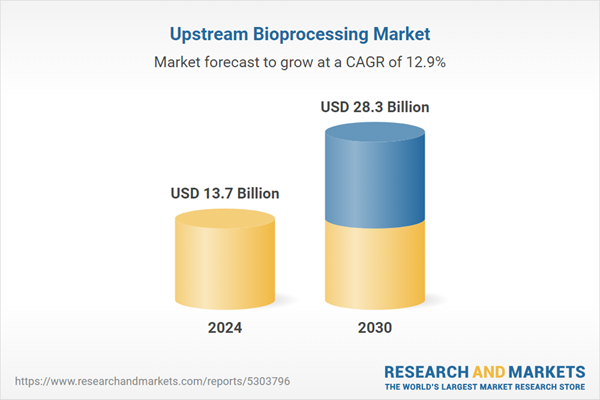
| Estimated Market Value ( USD | $ 13.7 Billion |
| Forecasted Market Value ( USD | $ 28.3 Billion |
| Compound Annual Growth Rate | 12.9% |
| Regions Covered | Global |